Abstract
The production of pharmaceutical compounds in plants is attracting increasing attention, as plant-based systems can be less expensive, safer, and more scalable than mammalian, yeast, bacterial, and insect cell expression systems. Here, we review the history and current status of plant-made pharmaceuticals. Producing pharmaceuticals in plants requires pairing the appropriate plant species with suitable transformation technology. Pharmaceuticals have been produced in tobacco, cereals, legumes, fruits, and vegetables via nuclear transformation, chloroplast transformation, transient expression, and transformation of suspension cell cultures. Despite this wide range of species and methods used, most such efforts have involved the nuclear transformation of tobacco. Tobacco readily generates large amounts of biomass, easily accepts foreign genes, and is amenable to stable gene expression via nuclear transformation. Although vaccines, antibodies, and therapeutic proteins have been produced in plants, such pharmaceuticals are not readily utilized by humans due to differences in glycosylation, and few such compounds have been approved due to a lack of clinical data. In addition, achieving an adequate immune response using plant-made pharmaceuticals can be difficult due to low rates of production compared to other expression systems. Various technologies have recently been developed to help overcome these limitations; however, plant systems are expected to increasingly become widely used expression systems for recombinant protein production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recombinant therapeutic proteins are transgenic proteins that are produced in a heterologous organism and are used to prevent or treat a human or animal disease. The first commercially available recombinant therapeutic protein was human insulin produced in Escherichia coli (Goeddel et al. 1979). Since then, as the technology has developed, other therapeutics, vaccines, and cytokines have been produced to protect people from infectious diseases and as cures for previously incurable diseases (Meyer et al. 2008). Unlike traditional pharmaceuticals produced by chemical synthesis, recombinant proteins are larger, with more complex structures. For example, aspirin (acetylsalicylic acid) is composed of 21 atoms, whereas a monoclonal antibody used as an anticancer agent is composed of 25,000 atoms and exhibits a complex structure (Otto et al. 2014). Given this complexity, systems to produce such recombinant proteins generally use living cells, including E. coli, yeast, insect, and mammalian cells (Thomas et al. 2002).
The production system is chosen based on the type of drug being produced. Prokaryotic production systems are used to produce proteins that have simple structures and lack post-translational modifications such as N-glycosylation, whereas eukaryotic production systems are used to produce proteins that have complex structures (such as antibodies) and post-translational modifications (Ferrer-Miralles et al. 2009). Bacterial expression systems were the first systems utilized to produce recombinant proteins, and they remain in use because bacterial cells are easy to handle and grow rapidly (Redwan 2007). However, bacterial systems also have significant disadvantages, including the presence of endotoxin and the production of proteins lacking glycosylation. For these reasons, and because most biopharmaceuticals require post-translational protein processing for function, eukaryotic yeast and mammalian cell expression systems have accounted for the majority of biopharmaceutical production (Butler 2003). Like bacteria, yeast cells are inexpensive to produce and grow quickly. In addition, they can produce glycosylated proteins (Gellison et al. 1992; Choi et al. 2003; Demain and Vaishnav 2009). Yeast cell disruption is difficult, however, due to the presence of the cell wall (Çelik and Çalık 2012). Recombinant proteins produced in insect cells can undergo proper folding and post-translational modification; however, insect cell systems are expensive and time-consuming to use (Fernandes et al. 2012). Because the mammalian cell culture system has the same advantages as the insect cell system and is optimized for human protein production, most recombinant protein production platforms are based on mammalian cells. Such systems have been approved for the production of a number of drugs (O'Flaherty et al. 2020). However, these systems are highly vulnerable to infection by animal pathogens, are difficult to scale up and require expensive culture conditions (Butler 2003).
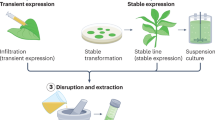
To overcome these disadvantages, plant production systems for biopharmaceuticals have been developed (Fig. 1) (Shanmugaraj et al. 2020). Plants are generally suitable for recombinant protein production. Recombinant proteins can be rapidly produced in plants via transient expression (Kapila et al. 1997). In addition, plant production systems cannot be infected by mammalian pathogens during protein production and are easy to scale up (Yusibov and Rabindran 2008). The production cost when using plant production system is estimated to be only 0.1% and 2–10% that when using mammalian cell culture and microbial fermentation systems, respectively (Giddings 2001). Moreover, their culture conditions are simple, and since plants are eukaryotic, the recombinant proteins undergo post-translational modification (Fischer et al. 1999a). However, the glycosylation process in plants is different from that in animal cell systems (Bosch et al. 2013). Although few plant-made recombinant proteins have been approved to date for use as therapeutic agents, these problems are being resolved with advances in biotechnology (Schillberg et al. 2019). The production of biopharmaceuticals in plants—known as molecular farming, a term introduced by Fischer et al. (1999a, b)—offers benefits such as scalability, speed, and improved safety compared with insect and mammalian cell expression systems. These features are desirable when resources are limited and could be helpful for the rapid production of vaccines against pandemic disease outbreaks or biochemical terrorism (Fischer et al. 1999a, b).
The US Food and Drug Administration approved the first vaccine produced in Nicotiana tabacum cell suspension culture expression system in 2006; this vaccine protects Newcastle disease virus in poultry (Vermij and Waltz 2006). In 2012, the first plant-made pharmaceutical (PMP) for humans was approved: a recombinant human glucocerebrosidase produced in a carrot (Daucus carota) cell suspension culture system. This enzyme is used to treat Gaucher’s disease, a hereditary lysosomal storage disorder caused by a mutation of the β-glucocerebrosidase gene (Fox 2012; Rosales-Mendoza and Tello-Olea 2015). The disease is characterized by enlargement of the liver and spleen, fatigue, and anemia (Tekoah et al. 2015). Since then, PMPs have been developed using various technologies, and the demand for these products is growing. The US Defense Advanced Research Projects Agency (DARPA), which is interested in the potential use of plant-derived vaccine production technology, has invested in both proof-of-concept operations and the development of commercial-scale facilities for plant-derived vaccine manufacturing. In particular, DARPA announced the design and operation of a facility that can produce tens of millions of doses of plant-derived vaccines via transient expression in Nicotiana benthamiana (Holtz et al. 2015). These vaccines can be ready for use within 1 month after the plants are treated with an appropriate vector, showing how quickly the production of “green vaccines” can be established using transient expression. By emphasizing how easily the upstream process could be expanded, the authors demonstrated the suitability of plant-derived vaccine production as a means to rapidly protect the population against new infectious diseases.
In this review, we discuss pharmaceutical proteins produced in plants. First, we look at the types of recombinant pharmaceutical proteins developed so far, including vaccines, antibodies, and therapeutic proteins. Next, we discuss tools and technologies for producing these proteins in plants, including production methods in various plant systems. Finally, we discuss the limitations of recombinant protein production using plant systems compared to mammalian, insect, yeast, and E. coli systems, as well as the current state of research and development aimed at solving these problems.
Three types of PMPs
High-value-added PMPs produced from plants include vaccines, antibodies, and therapeutic proteins. Clinical trials have been conducted on various PMPs, which are listed in Table 1 (https://clinicaltrials.gov, accessed on December 2022).
Vaccines
Vaccines confer acquired immunity against specific diseases or pathogens in animals, including humans. Vaccines are similar in structure to the antigen recognition sites of microbial pathogens that cause disease, but unlike pathogens, they are not pathogenic. Thus, vaccines activate the immune system, allowing it to quickly respond to subsequent infections with the same pathogen. The demand for plant-based vaccines has continuously increasing since the development of a vaccine against Newcastle virus in 2006 (Yusibov and Rabindran 2008; Shim et al. 2019). Research on various pathogens is being actively conducted, and some vaccines are currently being evaluated for safety and efficacy. In addition to general vaccines, which have attenuated pathogenicity, other types of vaccines are available, such as those made from inactivated viruses, recombinant protein subunits, and nucleic acids (Ellis 1999). In particular, a vaccine made from virus-like particles (VLPs; a type of recombinant protein subunit) is attracting attention (Noad and Roy 2003).
A VLP that is similar to a virus shape is not infectious because it does not contain viral genetic materials. Although VLPs lack pathogenicity, they contain the viral antigen(s) found on the outside of the capsid and are immunogenic (Murray 1988; Pumpens and Grens 2002). Representative plant-produced vaccines include vaccines against the hepatitis B virus (Huang et al. 2008), Newcastle disease virus (Guerrero-Andrade et al. 2006), influenza (D’Aoust et al. 2008; Kalthoff et al. 2010; Kanagarajan et al. 2012; Shoji et al. 2012; Firsov et al. 2015; Mbewana et al. 2015; Smith et al. 2020), porcine circovirus (Gunter et al. 2019), Zika virus (Yang et al. 2018), and poliovirus (Marsian et al. 2017). Recently, two types of plant-made COVID-19 vaccines were rapidly developed. Medicago's coronavirus VLP (CoVLP) is a vaccine that mimics the surface structure of the SARS-CoV-2 virus. Using a transient expression system in N. benthamiana, after the nucleotide sequence of the virus is obtained, a vaccine can be successfully produced within 8 weeks (Nosaki et al. 2021). This process is four- to eightfold faster than the currently used egg-production system for the influenza vaccine. Based on the promising results of the phase 1 clinical trial, phase 2/3 clinical trials were conducted and it was approved in Canada in 2022 (Ward et al. 2021; https://www.canada.ca/en/services/drugs-health-products.html, accessed on December 2022). Kentucky Bioprocessing (KBP) also produced a VLP-based vaccine in N. benthamiana. This vaccine candidate, KBP-201, is a chimeric VLP (cVLP) comprising the expressed SARS-CoV-2 receptor-binding domain (RBD) and a modified tobacco mosaic virus (TMV) (Maharjan et al. 2021). Since cVLPs consist of viruses that are not related to the antigens, they function as heterologous antigens; such molecules can induce a robust antibody response (Kushnir et al. 2012).
Antibodies
Antibodies are substances produced by the immune system in response to stimulation by antigens. Their basic structure comprises two light chains and two heavy chains, which form a Y shape through disulfide bonds. The amino acid sequences of antibodies vary depending on the type of antigen, allowing countless types of antibodies to be produced. In 1989, the production of single gamma or kappa immunoglobulin chains in tobacco was made possible for the first time (Hiatt et al. 1989); since then, numerous plant-made antibody therapeutics have been researched and developed. The plant-produced antibody drug ZMapp was developed by Mapp Pharmaceutical to treat Ebola virus (Pettitt et al. 2013). In addition, antibodies against cancer (So et al. 2013; Rattanapisit et al. 2019), human immunodeficiency virus (Floss et al. 2008; Sainsbury and Lomonossoff 2008; Holland et al. 2010), Zika, herpes (Diamos et al. 2020), and dengue (Dent et al. 2016) are currently being developed.
Therapeutic proteins
Cytokines are small glycoproteins involved in a variety of processes in the cell, including cell proliferation, differentiation, and migration. As cytokines are components of the human immune system, their most widely known role is to mediate signal transduction between adjacent or distant cells, causing positive or negative feedback cascades (Arai et al. 1990). The more than 100 cytokines identified to date are divided into several families, including hematopoietins, interferons, platelet-derived growth factors, tumor necrosis factors, tumor growth factors, and chemokines (Liles and Van Voorhis 1995). Cytokines can also be produced in animal cells, but due to the potential for contamination, efforts are focused on producing these molecules in plant systems. Using a coat protein (CP)-deficient bamboo mosaic virus (BaMV) vector, the expression level of human interferon-γ was higher compared to that of other viral vectors in N. benthamiana (Jiang et al. 2019). Moreover, the efficient removal of the affinity tag after purification during the production of human interleukin-6 reduced production costs.
The bleeding disorder hemophilia affects a small percentage of the world’s population, but it imposes a great burden on those who have it. In particular, more than half of hemophilia patients do not receive adequate treatment. Hemophilia is caused by a deficiency of factor VIII (FVIII), a blood coagulation factor (Mannucci and Tuddenham 2001). Because FVIII is known to undergo complex post-translational modification, research has focused on producing a therapeutic agent in eukaryotes. In plants, the FVIII domain was produced in N. tabacum chloroplasts and bioencapsulated in plant cells up to 0.4 mg/g in fresh leaves (Verma et al. 2010; Sherman et al. 2014). Furthermore, the entire FVIII factor was produced in the chloroplasts of lettuce (Lactuca sativa) at a level of 852 µg/g (Kwon et al. 2018). Another blood coagulation factor, a serine protease a-thrombin precursor (pFIIa) involved in fibrin formation, was co-expressed in N. benthamiana with Turnip Crinkle Virus coat protein (TCV-CP), which is known to interfere with post-transcriptional gene silencing (Laguia-Becher et al. 2019).
Human serum albumin (HSA), an abundant protein in the human body, accounts for more than half of the total protein content in plasma. Due to its strong binding properties, HSA acts as a carrier for hormones and metabolites and is also used to treat diseases or injuries such as hypovolemia, burns, and bleeding. HSA can be extracted from blood, but there has been considerable effort made to diversify its production due to the limited availability of donor human blood and the potential for blood-based infection. Studies to produce HSA using tobacco and rice (Oryza sativa) plants have been attempted (He et al. 2011). HSA expression in rice was successfully increased by knock-in of an HSA expression cassette at the locus encoding GluA1, a rice storage protein (Sedaghati et al. 2020).
Tools and techniques for PMP expression
Two major methods are used to produce recombinant proteins in plant systems: stable genetic transformation and transient gene expression (Table 2) (Potrykus 1991; Paul and Ma 2011). The method chosen depends on the plant species, target genome (nuclear or plastid), and gene characteristics (Christou 1996). Stable genetic transformation involves the stable production of proteins via the insertion of recombinant genes into the plant cell genomes (Horsch et al. 1985). This method has several advantages. In particular, it enables the stable, large-scale production of recombinant proteins, and allows for the production of multiple recombinant proteins. However, this method also has disadvantages: it is time-consuming and must be modified to ensure mass expression. Moreover, in nuclear transformation, since genes are randomly inserted into the nucleus, unstable expression or silencing of genes could occur due to positional effects (Fischer et al. 2004).
Transient gene expression can be used to produce a target protein more rapidly than stable genetic transformation. There are two major methods for transient gene expression: Agrobacterium-mediated infiltration and viral vector-based transient expression (Scholthof et al. 1996; Kapila et al. 1997). In both cases, stable integration of the transgene is not required.
Nuclear transformation
Among several methods for nuclear transformation, the most widely used nuclear transformation method is Agrobacterium-mediated plant transformation. In the case of plants which cannot be transformed with Agrobacterium, polyethylene glycol-mediated protoplast transformation method or biolistic transformation method can be used (Hayashimoto et al. 1990; Altpeter et al. 2005). Most recombinant proteins in plants generated to date have been produced through nuclear transformation, in which a gene of interest (GOI) is stably integrated into a chromosome in the nucleus of a plant cell. The natural plant pathogens Agrobacterium tumefaciens and Agrobacterium rhizogenes, which produce crown gall disease and hairy root disease, respectively, mediate indirect gene transfer during nuclear transformation (Anderson and Moore 1979). Agrobacterium tumefaciens carries a tumor-inducing (Ti) plasmid comprising a transfer DNA (T-DNA) region, a virulence (vir) gene, and an origin of replication. The T-DNA region contains auxin, cytokinin, and opine biosynthesis genes between the left border (LB) and right border (RB). Auxin and cytokinin are phytohormones that increase the size of plant cells and promote division. Opines, such as octopine and nopaline, are derivatives of various amino acids or sugar phosphates produced in host plants that serve as nutrients for Agrobacterium after infection. When an infection begins, the T-DNA region of the Ti-plasmid is transferred to the nucleus of the plant cell and then fuses with the plant genome. At this time, the vir genes are expressed to facilitate the transfer and integration of the T-DNA (Birch 1997).
Based on these mechanisms by which Agrobacterium tumefaciens mediates plant transformation, a T-DNA binary vector system was developed consisting of two autonomous-replicating disarmed Ti-plasmids working as helpers for T-DNA processing and transfer and a T-region-containing binary vector for cloning the GOI (Hoekema et al. 1983). The binary vector also contains multiple cloning sites, origins of replication that operate in both E. coli and Agrobacterium, and antibiotic selection markers that function in plants and E. coli. The helper plasmid contains vir genes that promote gene transfer and integration into plant cells (Özyiğit 2012). After Agrobacterium infection of plants, callus formation occurs, callus differentiation is induced, and shoots and roots are generated. The resulting transgenic plant thus becomes a host for recombinant protein production (De La Riva et al. 1998; Gelvin 2003; Van Montagu 2003). In many cases, the GOI is expressed under the control of a constitutive strong promoter such as the cauliflower mosaic virus (CaMV) 35S promoter to ensure copious production of the protein (Kay et al. 1987; Ma et al. 2003). In some cases, the GOI is expressed under the control of a seed-specific promoter, and the target protein accumulates in mature seeds. The advantage of this feature is that it is possible to obtain transgenic plants immediately by planting seeds that stably express the target gene (Twyman et al. 2003). The protein of interest could also be targeted to subcellular organelles such as plastids, the endoplasmic reticulum, or the vacuole. There are also various choices for post-translational modifications, as the proteins are retained in the endoplasmic reticulum or secreted into the apoplast (Schillberg et al. 2002).
Chloroplast transformation
In addition to inserting a GOI into the nucleus, the GOI can also be introduced into chloroplast chromosomes to express the recombinant protein in leaf tissue. The chloroplast is a membrane-bound organelle whose genetic characteristics closely resemble those of prokaryotes since chloroplasts are thought to have originated from cyanobacteria (Martin et al. 2002). Each mature leaf cell contains up to 100 chloroplasts, and each chloroplast contains ~ 100 copies of chloroplast genomic DNA. Thus, it provides a high gene copy number and leads to result in high expression of recombinant protein from integrated GOI in the chloroplast genome (Daniell et al. 2002). Chloroplast transformation is achieved by bombarding the leaf with gold particles coated with plastid DNA fragments containing the GOI and allowing the DNA to be integrated into the plastid genome via homologous recombination (Daniell et al. 1990). Homologous recombination allows the GOI to be inserted into a specific position in the chloroplast genome, and no post-transcriptional gene silencing has been observed (Daniell et al. 2005). In addition to bombardment, polyethylene glycol can be used to insert a GOI into the chloroplast genome (O'Neill et al. 1993; Díaz and Koop 2014). This simple, efficient method allows the simultaneous transformation of many samples, and the plant tissue shows a high survival rate, but the success rate is low compared to other methods.
Although transgenes are expressed at high levels in the chloroplast, a chloroplast-specific promoter is often used for optimization. For example, the psbA promoter exhibits higher translational activity than other promoters. Codon optimization of the target gene is also performed using the sequence of psbA to increase the translation rate (Kwon et al. 2016). However, plastids do not provide post-translational modification pathways such as glycosylation, making them unsuitable for the production of glycoproteins.
Transient expression
Unlike stably expressed transgenes, transiently expressed transgenes do not undergo chromosomal integration and are not affected by positional effects. Therefore, protein expression peaks within 18–48 h and is maintained for approximately 10 days (Kapila et al. 1997). Two major methods are used for transient expression: infiltration using A. tumefaciens or plant viral infection. Agro-infiltration is a simple method performed using the same binary vector used for stable transformation (Komori et al. 2007). The Agrobacterium culture is infiltrated in leaves using a needleless syringe or vacuum (Lee and Yang 2006; Simmons et al. 2009), and the leaves are harvested within 3–4 days post infiltration to extract proteins. However, agro-infiltrated leaves express low amounts of recombinant protein, and the use of this technology is limited to mass production platforms. Therefore, several methods have been used to increase protein expression following agro-infiltration. The expression level of a GOI increased up to sevenfold after the addition of 5-azacytidine (5AzaC), antioxidants, and surfactants to the system in N. benthamiana (Zhao et al. 2017). 5AzaC increases transgene expression by reducing DNA methylation, and antioxidants such as ascorbic acid, lipoic acid, and polyvinylpyrrolidone reduce the levels of reactive oxygen species generated during Agrobacterium infection and increase the efficiency of transformation. In addition, surfactants such as Tween-20, Triton X-100, and Silwet L-77 reduce the surface tension of plant cells to facilitate the invasion of Agrobacterium (Kuta and Tripathi 2005; Dan 2008).
Plant viruses such as TMV, potato virus X (PVX), BaMV, and cowpea mosaic virus (CPMV) are used to produce recombinant proteins because viruses express large amounts of specific proteins to ensure infection. Plant virus expression systems offer the advantage of rapid and high-level transgene expression (Lico et al. 2008). To construct a viral vector with high transgene expression, the coat and movement protein genes are removed from the viral genome and replaced with a target gene expression cassette. Because viral vectors produce large amounts of proteins under extreme conditions, it is possible to produce proteins at levels exceeding hundreds of mg/kg plant biomass using these vectors (Balke and Zeltins 2019).
Expression in suspension cell cultures
Plant suspension cell cultures are generated by growing individual cells or small aggregates derived from the breakdown of brittle callus pieces. Suspension cell culture is usually carried out in shaker flasks, and a bioreactor is used for subsequent scale-up. For recombinant protein production using suspension cells, tissues of cells co-cultured with A. tumefaciens or transgenic explants are used. Tobacco is one of the most widely used plant tissue sources due to its rapid division and easy transformation. For example, N. tabacum cv. Bright Yellow 2 (BY-2) cells derived from embryogenic root meristem cells are often used for suspension cell culture (Winicur et al. 1998). In addition to tobacco, alfalfa (Medicago sativa) (Pires et al. 2012), Arabidopsis thaliana (Plasson et al. 2009), soybean (Glycine max) (Ganapathi et al. 2007), tomato (Solanum lycopersicum) (Kwon et al. 2003), and carrot cells (Mikschofsky et al. 2009) are also used. The Israeli company Protalix BioTherapeutics used carrot suspension cells to produce the treatment for Gaucher’s disease mentioned previously, which represented the first approved PMP used to treat human disease.
The advantages of plant suspension cell culture systems for recombinant protein production are as follows. First, plant cells grow rapidly. For example, one batch culture of N. tabacum BY-2 cells takes approximately 1–2 weeks to generate, whereas the process from seeding to harvest of transgenic plants takes several months (Plasson et al. 2009). Second, scale-up is relatively simple because only the amounts of pre-culture and culture media need to be increased (Twyman et al. 2003). Third, recovery and purification of the product are simple. If the recombinant protein is secreted outside the cells, plant cell debris and by-products can easily be removed by filtration. In addition, since the recombinant protein is in a dissolved state in the medium, a separate extraction process is not required. Most products remain in the medium following filtration, and the subsequent purification yield is also high. However, scaling up a plant suspension cell culture system requires a complex process design, and recombinant protein production is expensive compared to that in stable or transient expression systems (Hellwig et al. 2004; Schillberg et al. 2013).
Host plants for PMP expression
Arabidopsis is an excellent model for plant science research due to its short generation time, high-density growth, and easy transformation (Meinke et al. 1998). However, Arabidopsis is not suitable for protein production due to its low biomass. Higher biomass and protein content of plants is advantageous for recombinant protein production since the relatively lower content of growth inhibitory factors and secondary metabolites (Fischer et al. 2004). Representative plants include tobacco, cereals, legumes, fruits, and vegetables.
Nicotiana spp.
N. benthamiana and N. tabacum have many desirable characteristics for the production of recombinant proteins, such as a fast growth rate, high biomass, and easy acceptance of foreign genes. Thus, the levels of recombinant proteins produced in tobacco are higher than in other crops (Fischer et al. 2004). For example, the expression level of green fluorescent protein in transformed tobacco exceeded 50% of total soluble proteins when a viral vector was used (Marillonnet et al. 2004). Also, because tobacco is not a food or feed crop, it is free from food- or feed-related contamination problems. Of course, many tobacco varieties contain high levels of toxic alkaloids, but low-alkaloid varieties exist that are used for the production of pharmaceutical proteins. When 52 Nicotiana varieties were evaluated, N. tabacum cv. I 64 showed the highest protein concentration, large amount of biomass, and small amount of alkaloids (Conley et al. 2011).
Cereals
Unlike leaf crops, protein expression in seeds has the advantage that it can be stored for a long time even at room temperature because proteins are stably accumulated (Lau and Sun 2009). In addition, since cereal seeds contain almost no phenolic compounds, the efficiency of downstream processes such as refining and analysis is high. These properties allow cereals containing therapeutics or vaccines to be administered orally with minimal processing. (Margolin et al. 2018). However, it is difficult to develop a suitable recombinant protein production system in the seeds of cereal plants because it is time-consuming to obtain seeds.
Legumes
Legumes such as alfalfa and soybeans have an advantage for protein production because their ability to fix atmospheric nitrogen lowers the need for chemical fertilizer (D'Aoust et al. 2004). Many studies have been conducted to produce recombinant proteins in alfalfa because it has a large dry biomass yield per area and can be harvested up to nine times per year (Ding et al. 2006; Joensuu et al. 2006; Aguirreburualde et al. 2013). For example, lactoferrin has been produced in alfalfa (Stefanova et al. 2013). In addition, soybean has a high protein content in seeds (> 40%) as compared to other crops, which is beneficial for recombinant protein production (Hudson et al. 2011). In addition, soybeans have an excellent ability to stably store protein. For example, the recombinant protein remained stable in soybean seeds at room temperature even after 7 years (Cunha et al. 2010). However, since soybean seeds contain large amounts of oil, the efficiency of downstream processes such as refining and analysis is not high (Stoger et al. 2005).
Fruits and vegetables
The greatest advantage of using fruits and vegetables for protein expression is that the tissue can be consumed uncooked, unprocessed, or partially processed. This is very important for the production of recombinant proteins such as vaccines, antibodies, and nutraceuticals. Potato (Solanum tuberosum) represents a major system for vaccine production; studies have been conducted on vaccine production for infectious bronchitis virus and hepatitis B virus in potatoes (Zhou et al. 2004; Thanavala et al. 2005). Tomatoes, bananas, and strawberries could also be used for recombinant protein production; unlike potatoes, tomatoes, bananas, and strawberries that produce recombinant protein can be eaten raw and used as 'edible vaccines'. Tomatoes also are delicious and have a higher biomass yield than potatoes. A recent study highlighted the possibility of generating a colorectal cancer vaccine by producing an rGAA-733 antigen in tomatoes (Park et al. 2022). Bananas are another attractive edible vaccine production platform, because they can be grown in wide areas and are enjoyed by most people. For example, bananas producing hepatitis B surface antigen and cholera toxin B subunit have been reported (Kumar et al. 2005; Renuga et al. 2010). In addition, strawberries that produce canine interferon-α were developed in Japan and commercialized in 2014, proving their effectiveness in preventing periodontal disease in dogs (Tabayashi and Matsumura 2014).
Duckweed
Duckweed is a perennial monocotyledonous plant belonging to the Lemnaceae that lives on the water surface in rice fields or ponds. A small oval winter bud from the mother plant sinks into the water in the autumn, stays dormant in the water in the winter, and rises to the water surface in the spring of the following year to undergo asexual reproduction (Landolt and Kandeler 1987). Duckweed grows rapidly and can double in one day, and its culture and propagation are simple (Vunsh et al. 2007; Firsov et al. 2015). In addition, up to 45% of the plant body is made up of protein, and it is relatively resistant to contamination (Escobar and Escobar 2017). Duckweed has several advantages as a PMP platform: First, scale-up is easy due to the low cost of culture medium for duckweed. Second, since it does not produce pollen, duckweed is safe from concerns about gene transfer into the ecosystem. Finally, duckweed is edible. These advantages make duckweed an ideal protein production platform for oral delivery (Popov et al. 2006; Rival et al. 2008).
Moss
Physcomitrium patens (spreading earthmoss) can grow rapidly in an inorganic medium without phytohormones or vitamins. Genetic studies have revealed that most mosses exist as haploids during the growing season, pointing to their ease of transformation (Reski and Cove 2004). P. patens shows high stability as a protein production platform because it undergoes a complex post-translational modification process during protein expression. This can be an advantage for the production of exogenous recombinant proteins because it allows stable protein production (von Stackelberg et al. 2006; Parsons et al. 2013).
Conclusions and future perspectives
Plant expression systems for recombinant protein production have several specific advantages. However, their application in recombinant protein production must overcome major barriers such as a lack of regulatory approval. PMPs are currently used primarily for diagnostic purposes and as veterinary medicines. Although it is difficult to obtain approval for PMP as a human medical protein, it will be necessary to secure a basis for approval for the production of recombinant human medical protein in the future. These systems also have some disadvantages, such as plant-specific glycosylation patterns differing from those in animal cells, low yields for recombinant protein production, and complex transgenic plant management practices, but solutions to these problems are possible with the development of new technologies.
Protein glycosylation is an essential post-translational modification that occurs in all eukaryotes. The early steps of this process are well conserved in plants, animals, and yeast, but late N-glycan maturation in the Golgi apparatus varies considerably. Therefore, it will be important to control the glycosylation of target proteins in plants (Koprivova et al. 2004; Strasser et al. 2004). Indeed, Protalix BioTherapeutics increased the therapeutic efficacy of ELELYSO™ by adding mannose to N-glycan (Tekoah et al. 2015). A recent study demonstrated a technique for removing xylose and fucose from N-glycan in plants (Jansing et al. 2019). In the future, we expect that this technology will be further developed to freely control whether N-glycan residues are attached to target proteins in plants.
A recently developed type of N. tabacum does not produce alkaloid nicotine (Schachtsiek and Stehle 2019); this advance is expected to help increase the yield of recombinant proteins in this system. Other studies have been conducted to overcome the yield-related difficulties in inducing immunogenicity. For example, a split-intein SpyTag/SpyCatcher (ST/SC) conjugation system is a complementary peptide that can bind over a wide range of temperatures and pH values, allowing it to be used for the efficient binding of two proteins (Zakeri et al. 2012). VLPs from West Nile virus were efficiently formed using this system (Stander et al. 2021). In addition, several antigen-display technologies, such as bacteria-like particles (BLPs) have been developed (van Roosmalen et al. 2006; Bosma et al. 2006; Buist et al. 2008). The BLP system is used for antigen display by inactivating Lactococcus bacteria, which are generally recognized as safe. The display capacity was improved by binding mCor1 homotrimer and lysM to the antigen protein. In a recent study, the immunogenicity of a vaccine for avian influenza virus was significantly improved using this technology (Song et al. 2021). Such improvements should lead to the widespread adoption of PMPs in the future.
References
Aguirreburualde MSP, Gómez MC, Ostachuk A, Wolman F, Albanesi G, Pecora A, Odeon A, Ardila F, Escribano JM, Santos MJD (2013) Efficacy of a BVDV subunit vaccine produced in alfalfa transgenic plants. Vet Immunol Immunopathol 151(3–4):315–324. https://doi.org/10.1016/j.vetimm.2012.12.004
Altpeter F, Baisakh N, Beachy R, Bock R, Capell T, Christou P, Daniell H, Datta K, Datta S, Dix PJ, Fauquet C, Huang N, Kohli A, Mooibroek H, Nicholson L, Nguyen TT, Nugent G, Raemakers K, Romano A, Somers DA, Stoger E, Taylor N, Visser R (2005) Particle bombardment and the genetic enhancement of crops: myths and realities. Mol Breed 15:305–327. https://doi.org/10.1007/s11032-004-8001-y
Anderson AR, Moore LW (1979) Host specificity in the genus Agrobacterium. Phytopathol 69(4):320-323
Arai K-i, Lee F, Miyajima A, Miyatake S, Arai N, Yokota T (1990) Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem 59(1):783–836. https://doi.org/10.1146/annurev.bi.59.070190.004031
Balke I, Zeltins A (2019) Use of plant viruses and virus-like particles for the creation of novel vaccines. Adv Drug Del Rev 145:119–129. https://doi.org/10.1016/j.addr.2018.08.007
Birch RG (1997) Plant tramsformation: problems and strategies for practical application. Annu Rev Plant Biol 48(1):297–326. https://doi.org/10.1146/annurev.arplant.48.1.297
Bosch D, Castilho A, Loos A, Schots A, Steinkellner H (2013) N-glycosylation of plant-produced recombinant proteins. Curr Pharm Des 19(31):5503–5512. https://doi.org/10.2174/138161281139310006
Bosma T, Kanninga R, Neef J, Audouy SA, van Roosmalen ML, Steen A, Buist G, Kok J, Kuipers OP, Robillard G (2006) Novel surface display system for proteins on non-genetically modified gram-positive bacteria. Appl Environ Microbiol 72(1):880–889. https://doi.org/10.1128/AEM.72.1.880-889.2006
Buist G, Steen A, Kok J, Kuipers OP (2008) LysM, a widely distributed protein motif for binding to (peptido) glycans. Mol Microbiol 68(4):838–847. https://doi.org/10.1111/j.1365-2958.2008.06211.x
Butler M (2003) Animal cell culture and technology. Taylor & Francis, pp 1–29. https://doi.org/10.4324/9780203427835
Çelik E, Çalık P (2012) Production of recombinant proteins by yeast cells. Biotechnol Adv 30(5):1108–1118. https://doi.org/10.1016/j.biotechadv.2011.09.011
Choi BK, Bobrowicz P, Davidson RC, Hamilton SR, Kung DH, Li H, Miele RG, Nett JH, Wildt S, Gerngross TU (2003) Use of combinatorial genetic libraries to humanize N-linked glycosylation in the yeast Pichia pastoris. Proc Natl Acad Sci USA 100(9):5022–5027. https://doi.org/10.1073/pnas.0931263100
Christou P (1996) Transformation technology. Trends Plant Sci 1(12):423–431. https://doi.org/10.1016/S1360-1385(96)10047-9
Conley AJ, Zhu H, Le LC, Jevnikar AM, Lee BH, Brandle JE, Menassa R (2011) Recombinant protein production in a variety of Nicotiana hosts: a comparative analysis. Plant Biotechnol J 9(4):434–444. https://doi.org/10.1111/j.1467-7652.2010.00563.x
Cunha N, Araújo A, Leite A, Murad A, Vianna G, Rech E (2010) Correct targeting of proinsulin in protein storage vacuoles of transgenic soybean seeds. Genet Mol Res 9(2):1163–1170. https://doi.org/10.4238/vol9-2gmr849
D’Aoust MA, Lavoie PO, Couture MMJ, Trépanier S, Guay JM, Dargis M, Mongrand S, Landry N, Ward BJ, Vézina LP (2008) Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol J 6(9):930–940. https://doi.org/10.1111/j.1467-7652.2008.00384.x
Dan Y (2008) Biological functions of antioxidants in plant transformation. In Vitro Cell Dev Biol - Plant 44(3):149–161. https://doi.org/10.1007/s11627-008-9110-9
Daniell H, Vivekananda J, Nielsen BL, Ye GN, Tewari KK, Sanford JC (1990) Transient foreign gene expression in chloroplasts of cultured tobacco cells after biolistic delivery of chloroplast vectors. Proc Natl Acad Sci USA 87(1):88–92. https://doi.org/10.1073/pnas.87.1.88
Daniell H, Khan MS, Allison L (2002) Milestones in chloroplast genetic engineering: an environmentally friendly era in biotechnology. Trends Plant Sci 7(2):84–91. https://doi.org/10.1016/S1360-1385(01)02193-8
Daniell H, Kumar S, Dufourmantel N (2005) Breakthrough in chloroplast genetic engineering of agronomically important crops. Trends Biotechnol 23(5):238–245. https://doi.org/10.1016/j.tibtech.2005.03.008
D’Aoust M-A, Busse U, Martel M, Lerouge P, Levesque D, Vézina L-P (2004) Perennial plants as a production system for pharmaceuticals. Handb Plant Biotechnol. https://doi.org/10.1002/0470869143.kc043
De La Riva GA, González-Cabrera J, Vázquez-Padrón R, Ayra-Pardo C (1998) Agrobacterium tumefaciens: a natural tool for plant transformation. Electron J Biotechnol 1(3):24–25. https://doi.org/10.4067/S0717-34581998000300002
Demain AL, Vaishnav P (2009) Production of recombinant proteins by microbes and higher organisms. Biotechnol Adv 27(3):297–306. https://doi.org/10.1016/j.biotechadv.2009.01.008
Dent M, Hurtado J, Paul AM, Sun H, Lai H, Yang M, Esqueda A, Bai F, Steinkellner H, Chen Q (2016) Plant-produced anti-dengue virus monoclonal antibodies exhibit reduced antibody-dependent enhancement of infection activity. J Gen Virol 97(12):3280–3290. https://doi.org/10.1099/jgv.0.000635
Diamos AG, Hunter JGL, Pardhe MD, Rosenthal SH, Sun H, Foster BC, DiPalma MP, Chen Q, Mason HS (2020) High level production of monoclonal antibodies using an optimized plant expression system. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2019.00472
Díaz AH, Koop H-U (2014) Nicotiana tabacum: PEG-mediated plastid transformation. In: Maliga P (ed) Chloroplast biotechnology: methods and protocols. Humana Press, Totowa, pp 165–175. https://doi.org/10.1007/978-1-62703-995-6_9
Ding S-H, Huang L-Y, Wang Y-D, Sun H-C, Xiang Z-H (2006) High-level expression of basic fibroblast growth factor in transgenic soybean seeds and characterization of its biological activity. Biotechnol Lett 28(12):869–875. https://doi.org/10.1007/s10529-006-9018-6
Ellis RW (1999) New technologies for making vaccines. Vaccine 17(13–14):1596–1604. https://doi.org/10.1016/S0264-410X(98)00416-2
Escobar CM, Escobar AC (2017) Duckweed: a tiny aquatic plant with enormous potential for bioregenerative life support systems. Int Conf Environ Syst 281:1-9
Fernandes F, Vidigal J, Dias MM, Prather KLJ, Coroadinha AS, Teixeira AP, Alves PM (2012) Flipase-mediated cassette exchange in Sf9 insect cells for stable gene expression. Biotechnol Bioeng 109(11):2836–2844. https://doi.org/10.1002/bit.24542
Ferrer-Miralles N, Domingo-Espín J, Corchero JL, Vázquez E, Villaverde A (2009) Microbial factories for recombinant pharmaceuticals. Microb Cell Factor 8(1):1–8. https://doi.org/10.1186/1475-2859-8-17
Firsov A, Tarasenko I, Mitiouchkina T, Ismailova N, Shaloiko L, Vainstein A, Dolgov S (2015) High-yield expression of M2e peptide of avian influenza virus H5N1 in transgenic duckweed plants. Mol Biotechnol 57(7):653–661. https://doi.org/10.1007/s12033-015-9855-4
Fischer R, Drossard J, Commandeur U, Schillberg S, Emans N (1999a) Towards molecular farming in the future: moving from diagnostic protein and antibody production in microbes to plants. Biotechnol Appl Biochem 30(2):101–108. https://doi.org/10.1111/j.1470-8744.1999.tb00898.x
Fischer R, Liao Y-C, Hoffmann K, Schillberg S, Emans N (1999b) Molecular farming of recombinant antibodies in plants. Biol Chem 380(7–8):825–839. https://doi.org/10.1515/BC.1999.102
Fischer R, Stoger E, Schillberg S, Christou P, Twyman RM (2004) Plant-based production of biopharmaceuticals. Curr Opin Plant Biol 7(2):152–158. https://doi.org/10.1016/j.pbi.2004.01.007
Floss DM, Sack M, Stadlmann J, Rademacher T, Scheller J, Stöger E, Fischer R, Conrad U (2008) Biochemical and functional characterization of anti-HIV antibody–ELP fusion proteins from transgenic plants. Plant Biotechnol J 6(4):379–391. https://doi.org/10.1111/j.1467-7652.2008.00326.x
Fox JL (2012) First plant-made biologic approved. Nat Biotechnol 30(6):472–473. https://doi.org/10.1038/nbt0612-472
Ganapathi T, Kumar GS, Srinivas L, Revathi C, Bapat V (2007) Analysis of the limitations of hepatitis B surface antigen expression in soybean cell suspension cultures. Plant Cell Rep 26(9):1575–1584. https://doi.org/10.1007/s00299-007-0379-7
Gellison G, Janowicz ZA, Weydemann U, Melber K, Strasser AWM, Hollenberg CP (1992) High-level expression of foreign genes in Hansenula polymorpha. Biotech Adv 10:179–189. https://doi.org/10.1016/0734-9750(92)90002-Q
Gelvin SB (2003) Agrobacterium-mediated plant transformation: the biology behind the “gene-jockeying” tool. Microbiol Mol Rev 67(1):16–37. https://doi.org/10.1128/MMBR.67.1.16-37.2003
Giddings G (2001) Transgenic plants as protein factories. Curr Opin Biotechnol 12:450–454. https://doi.org/10.1016/s0958-1669(00)00244-5
Goeddel DV, Kleid DG, Bolivar F, Heyneker HL, Yansura DG, Crea R, Hirose T, Kraszewski A, Itakura K, Riggs AD (1979) Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci USA 76(1):106–110. https://doi.org/10.1073/pnas.76.1.106
Guerrero-Andrade O, Loza-Rubio E, Olivera-Flores T, Fehérvári-Bone T, Gómez-Lim MA (2006) Expression of the Newcastle disease virus fusion protein in transgenic maize and immunological studies. Transgenic Res 15(4):455–463. https://doi.org/10.1007/s11248-006-0017-0
Gunter CJ, Regnard GL, Rybicki EP, Hitzeroth II (2019) Immunogenicity of plant-produced porcine circovirus-like particles in mice. Plant Biotechnol J 17(9):1751–1759. https://doi.org/10.1111/pbi.13097
Hayashimoto A, Li Z, Murai N (1990) A polyethylene glycol-mediated protoplast transformation system for production of fertile transgenic rice plants. Plant Physiol 93(3):857–863. https://doi.org/10.1104/pp.93.3.857
He Y, Ning T, Xie T, Qiu Q, Zhang L, Sun Y, Jiang D, Fu K, Yin F, Zhang W, Shen L, Wang H, Li J, Lin Q, Sun Y, Li H, Zhu Y, Yang D (2011) Large-scale production of functional human serum albumin from transgenic rice seeds. Proc Natl Acad Sci USA 108(47):19078–19083. https://doi.org/10.1073/pnas.1109736108
Hellwig S, Drossard J, Twyman RM, Fischer R (2004) Plant cell cultures for the production of recombinant proteins. Nat Biotechnol 22(11):1415–1422. https://doi.org/10.1038/nbt1027
Hiatt A, Caffferkey R, Bowdish K (1989) Production of antibodies in transgenic plants. Nature 342(6245):76–78. https://doi.org/10.1038/342076a0
Hoekema A, Hirsch PR, Hooykaas PJJ, Schilperoort RA (1983) A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303(5913):179–180. https://doi.org/10.1038/303179a0
Holland T, Sack M, Rademacher T, Schmale K, Altmann F, Stadlmann J, Fischer R, Hellwig S (2010) Optimal nitrogen supply as a key to increased and sustained production of a monoclonal full-size antibody in BY-2 suspension culture. Biotechnol Bioeng 107(2):278–289. https://doi.org/10.1002/bit.22800
Holtz BR, Berquist BR, Bennett LD, Kommineni VJM, Munigunti RK, White EL, Wilkerson DC, Wong K-YI, Ly LH, Marcel S (2015) Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnol J 13(8):1180–1190. https://doi.org/10.1111/pbi.12469
Horsch RB, Fry JE, Hoffmann NL, Wallroth M, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227(4691):1229–1231. https://doi.org/10.1126/science.227.4691.1229
Huang Z, LePore K, Elkin G, Thanavala Y, Mason HS (2008) High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system. Plant Biotechnol J 6(2):202–209. https://doi.org/10.1111/j.1467-7652.2007.00316.x
Hudson LC, Bost KL, Piller KJ (2011) Optimizing recombinant protein expression in soybean. In: Soybean-molecular aspects of breeding InTech Open, pp 19–42
Jansing J, Sack M, Augustine SM, Fischer R, Bortesi L (2019) CRISPR/Cas9-mediated knockout of six glycosyltransferase genes in Nicotiana benthamiana for the production of recombinant proteins lacking β-1,2-xylose and core α-1,3-fucose. Plant Biotechnol J 17(2):350–361. https://doi.org/10.1111/pbi.12981
Jiang MC, Hu CC, Lin NS, Hsu YH (2019) Production of human IFNγ protein in Nicotiana benthamiana plant through an enhanced expression system based on bamboo mosaic virus. Viruses. https://doi.org/10.3390/v11060509
Joensuu J, Verdonck F, Ehrström A, Peltola M, Siljander-Rasi H, Nuutila A-M, Oksman-Caldentey K-M, Teeri T, Cox E, Goddeeris B (2006) F4 (K88) fimbrial adhesin FaeG expressed in alfalfa reduces F4+ enterotoxigenic Escherichia coli excretion in weaned piglets. Vaccine 24(13):2387–2394. https://doi.org/10.1016/j.vaccine.2005.11.056
Kalthoff D, Giritch A, Geisler K, Bettmann U, Klimyuk V, Hehnen H-R, Gleba Y, Beer M (2010) Immunization with plant-expressed hemagglutinin protects chickens from lethal highly pathogenic avian influenza virus H5N1 challenge infection. J Virol 84(22):12002–12010. https://doi.org/10.1128/JVI.00940-10
Kanagarajan S, Tolf C, Lundgren A, Waldenström J, Brodelius PE (2012) Transient expression of hemagglutinin antigen from low pathogenic avian influenza A (H7N7) in Nicotiana benthamiana. PLoS ONE 7(3):e33010. https://doi.org/10.1371/journal.pone.0033010
Kapila J, De Rycke R, Van Montagu M, Angenon G (1997) An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci 122(1):101–108. https://doi.org/10.1016/S0168-9452(96)04541-4
Kay R, Chan A, Daly M, McPherson J (1987) Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236(4806):1299–1302. https://doi.org/10.1126/science.236.4806.1299
Komori T, Imayama T, Kato N, Ishida Y, Ueki J, Komari T (2007) Current status of binary vectors and superbinary vectors. Plant Physiol 145(4):1155–1160. https://doi.org/10.1104/pp.107.105734
Koprivova A, Stemmer C, Altmann F, Hoffmann A, Kopriva S, Gorr G, Reski R, Decker EL (2004) Targeted knockouts of Physcomitrella lacking plant-specific immunogenic N-glycans. Plant Biotechnol J 2(6):517–523. https://doi.org/10.1111/j.1467-7652.2004.00100.x
Kumar GB, Ganapathi TR, Revathi CJ, Srinivas L, Bapat VA (2005) Expression of hepatitis B surface antigen in transgenic banana plants. Planta 222(3):484–493. https://doi.org/10.1007/s00425-005-1556-y
Kushnir N, Streatfield SJ, Yusibov V (2012) Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 31(1):58–83. https://doi.org/10.1016/j.vaccine.2012.10.083
Kuta DD, Tripathi L (2005) Agrobacterium-induced hypersensitive necrotic reaction in plant cells: a resistance response against Agrobacterium-mediated DNA transfer. Afr J Biotechnol 4(8):752–757. https://doi.org/10.5897/AJB2005.000-3149
Kwon T-H, Kim Y-S, Lee J-H, Yang M-S (2003) Production and secretion of biologically active human granulocyte-macrophage colony stimulating factor in transgenic tomato suspension cultures. Biotechnol Lett 25(18):1571–1574. https://doi.org/10.1023/a:1025409927790
Kwon K-C, Chan H-T, León IR, Williams-Carrier R, Barkan A, Daniell H (2016) Codon optimization to enhance expression yields insights into chloroplast translation. Plant Physiol 172(1):62–77. https://doi.org/10.1104/pp.16.00981
Kwon KC, Sherman A, Chang WJ, Kamesh A, Biswas M, Herzog RW, Daniell H (2018) Expression and assembly of largest foreign protein in chloroplasts: oral delivery of human FVIII made in lettuce chloroplasts robustly suppresses inhibitor formation in haemophilia A mice. Plant Biotechnol J 16(6):1148–1160. https://doi.org/10.1111/pbi.12859
Laguia-Becher M, Zaldúa Z, Xu W, Marconi PL, Velander W, Alvarez MA (2019) Co-expressing Turnip Crinkle Virus-coat protein with the serine protease α-thrombin precursor (pFIIa) in Nicotiana benthamiana Domin. In Vitro Cell Dev Biol Plant 55(1):88–98
Landolt E, Kandeler R (1987) Biosystematic investigations in the family of duckweeds (Lemnaceae), Vol. 4: the family of Lemnaceae-a monographic study, Vol. 2 (phytochemistry, physiology, application, bibliography). Veroeffentlichungen des Geobotanischen Instituts der ETH, Stiftung Ruebel
Lau OS, Sun SSM (2009) Plant seeds as bioreactors for recombinant protein production. Biotechnol Adv 27(6):1015–1022. https://doi.org/10.1016/j.biotechadv.2009.05.005
Lee MW, Yang Y (2006) Transient expression assay by agroinfiltration of leaves. Methods Mol Biol 323:225–229. https://doi.org/10.1385/1-59745-003-0:225
Lico C, Chen Q, Santi L (2008) Viral vectors for production of recombinant proteins in plants. J Cell Physiol 216(2):366–377. https://doi.org/10.1002/jcp.21423
Liles WC, Van Voorhis WC (1995) Nomenclature and biologic significance of cytokines involved in inflammation and the host immune response. J Infect Dis 172(6):1573–1580. https://doi.org/10.1093/infdis/172.6.1573
Ma JKC, Drake PMW, Christou P (2003) The production of recombinant pharmaceutical proteins in plants. Nat Rev Genet 4(10):794–805. https://doi.org/10.1038/nrg1177
Maharjan PM, Cheon J, Jung J, Kim H, Lee J, Song M, Jeong GU, Kwon Y, Shim B, Choe S (2021) Plant-expressed receptor binding domain of the SARS-CoV-2 spike protein elicits humoral immunity in mice. Vaccines 9(9):978. https://doi.org/10.3390/vaccines9090978
Mannucci PM, Tuddenham EG (2001) The hemophilias–from royal genes to gene therapy. N Engl J Med 344(23):1773–1779. https://doi.org/10.1056/nejm200106073442307
Margolin E, Chapman R, Williamson AL, Rybicki EP, Meyers AE (2018) Production of complex viral glycoproteins in plants as vaccine immunogens. Plant Biotechnol J 16(9):1531–1545. https://doi.org/10.1111/pbi.12963
Marillonnet S, Giritch A, Gils M, Kandzia R, Klimyuk V, Gleba Y (2004) In planta engineering of viral RNA replicons: efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc Natl Acad Sci USA 101(18):6852. https://doi.org/10.1073/pnas.0400149101
Marsian J, Fox H, Bahar MW, Kotecha A, Fry EE, Stuart DI, Macadam AJ, Rowlands DJ, Lomonossoff GP (2017) Plant-made polio type 3 stabilized VLPs—a candidate synthetic polio vaccine. Nat Commun 8(1):1–9. https://doi.org/10.1038/s41467-017-00090-w
Martin W, Rujan T, Richly E, Hansen A, Cornelsen S, Lins T, Leister D, Stoebe B, Hasegawa M, Penny D (2002) Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc Natl Acad Sci USA 99(19):12246–12251. https://doi.org/10.1073/pnas.182432999
Mbewana S, Mortimer E, Pêra FF, Hitzeroth II, Rybicki EP (2015) Production of H5N1 influenza virus matrix protein 2 ectodomain protein bodies in tobacco plants and in insect cells as a candidate universal influenza vaccine. Front Bioeng Biotechnol 3:197. https://doi.org/10.3389/fbioe.2015.00197
Meinke DW, Cherry JM, Dean C, Rounsley SD, Koornneef M (1998) Arabidopsis thaliana: a model plant for genome analysis. Science 282(5389):662–682. https://doi.org/10.1126/science.282.5389.662
Meyer H, Brass J, Jungo C, Klein J, Wenger J, Mommers R (2008) An emerging star for therapeutic and catalytic protein production. Bioprocess Int 6:10
Mikschofsky H, Hammer M, Schmidtke J, König P, Keil G, Schirrmeier H, Schmidt K, Broer I (2009) Optimization of growth performance of freshly induced carrot suspensions concerning PMP production. In Vitro Cell Dev Biol Plant 45(6):740–749. https://doi.org/10.1007/s11627-008-9189-z
Murray K (1988) Application of recombinant DNA techniques in the development of viral vaccines. Vaccine 6(2):164–174. https://doi.org/10.1016/s0264-410x(88)80022-7
Noad R, Roy P (2003) Virus-like particles as immunogens. Trends Microbiol 11(9):438–444. https://doi.org/10.1016/S0966-842X(03)00208-7
Nosaki S, Hoshikawa K, Ezura H, Miura K (2021) Transient protein expression systems in plants and their applications. Plant Biotechnol (tokyo) 38(3):297–304. https://doi.org/10.5511/plantbiotechnology.21.0610a
O’Flaherty R, Bergin A, Flampouri E, Mota LM, Obaidi I, Quigley A, Xie Y, Butler M (2020) Mammalian cell culture for production of recombinant proteins: a review of the critical steps in their biomanufacturing. Biotechnol Adv 43:107552. https://doi.org/10.1016/j.biotechadv.2020.107552
O’Neill C, Horvath GV, Horvath E, Dix PJ, Medgyesy P (1993) Chloroplast transformation in plants: polyethylene glycol (PEG) treatment of protoplasts is an alternative to biolistic delivery systems. Plant J 3(5):729–738. https://doi.org/10.1111/j.1365-313X.1993.00729.x
Otto R, Santagostino A, Schrader U (2014) Rapid growth in biopharma: challenges and opportunities. McKinsey & Company
Özyiğit İİ (2012) Agrobacterium tumefaciens and its use in plant biotechnology. In: Ashraf M, Öztürk M, Ahmad MSA, Aksoy A (eds) Crop production for agricultural improvement. Springer Netherlands, Dordrecht, pp 317–361. https://doi.org/10.1007/978-94-007-4116-4_12
Park SH, Ji K-Y, Park SY, Kim HM, Ma SH, Do JH, Kang H, Kang HS, Oh D-B, Shim JS (2022) Immunotherapeutic effects of recombinant colorectal cancer antigen produced in tomato fruits. Sci Rep 12(1):1–10. https://doi.org/10.1038/s41598-022-13839-1
Parsons J, Altmann F, Graf M, Stadlmann J, Reski R, Decker EL (2013) A gene responsible for prolyl-hydroxylation of moss-produced recombinant human erythropoietin. Sci Rep 3(1):1–8. https://doi.org/10.1038/srep03019
Paul M, Ma JKC (2011) Plant-made pharmaceuticals: leading products and production platforms. Biotechnol Appl Biochem 58:58–67. https://doi.org/10.1002/bab.6
Pettitt J, Zeitlin L, Kim DH, Working C, Johnson JC, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, Pauly MH, Whaley KJ, Ingram MF, Zovanyi A, Heinrich M, Piper A, Zelko J, Olinger GG (2013) Therapeutic intervention of ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci Transl Med 5(199):199ra113. https://doi.org/10.1126/scitranslmed.3006608
Pires AS, Rosa S, Castanheira S, Fevereiro P, Abranches R (2012) Expression of a recombinant human erythropoietin in suspension cell cultures of Arabidopsis, tobacco and Medicago. Plant Cell Tissue Organ Cult 110(1):171–181. https://doi.org/10.1007/s11240-012-0141-x
Plasson C, Michel R, Lienard D, Saint-Jore-Dupas C, Sourrouille C, de March GG, Gomord V (2009) Production of recombinant proteins in suspension–cultured plant cells. Methods Mol Biol 483:145-161 https://doi.org/10.1007/978-1-59745-407-0_9
Popov SV, Golovchenko VV, Ovodova RG, Smirnov VV, Khramova DS, Popova GY, Ovodov YS (2006) Characterisation of the oral adjuvant effect of lemnan, a pectic polysaccharide of Lemna minor L. Vaccine 24(26):5413–5419. https://doi.org/10.1016/j.vaccine.2006.03.076
Potrykus I (1991) Gene transfer to plants: assessment of published approaches and results. Annu Rev Plant Physiol 42(1):205–225
Pumpens P, Grens E (2002) Artificial genes for chimeric virus-like particles. Artif DNA Methods Appl 249:327
Rattanapisit K, Phakham T, Buranapraditkun S, Siriwattananon K, Boonkrai C, Pisitkun T, Hirankarn N, Strasser R, Abe Y, Phoolcharoen W (2019) Structural and in vitro functional analyses of novel plant-produced anti-human PD1 antibody. Sci Rep 9(1):1–10. https://doi.org/10.1038/s41598-019-51656-1
Redwan E-RM (2007) Cumulative updating of approved biopharmaceuticals. Hum Antib 16(3–4):137–158
Renuga G, Saravanan R, Thandapani AB, Arumugam K (2010) Expression of cholera toxin B subunit in Banana callus culture. J Pharm Sci & Res 2(1):26
Reski R, Cove DJ (2004) Physcomitrella patens. Curr Biol 14(7):R261–R262
Rival S, Wisniewski J-P, Langlais A, Kaplan H, Freyssinet G, Vancanneyt G, Vunsh R, Perl A, Edelman M (2008) Spirodela (duckweed) as an alternative production system for pharmaceuticals: a case study, aprotinin. Transgenic Res 17(4):503–513. https://doi.org/10.1007/s11248-007-9123-x
Rosales-Mendoza S, Tello-Olea MA (2015) Carrot cells: a pioneering platform for biopharmaceuticals production. Mol Biotechnol 57(3):219–232. https://doi.org/10.1007/s12033-011-9837-y
Sainsbury F, Lomonossoff GP (2008) Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol 148(3):1212–1218. https://doi.org/10.1104/pp.108.126284
Schachtsiek J, Stehle F (2019) Nicotine-free, nontransgenic tobacco (Nicotiana tabacum l.) edited by CRISPR-Cas9. Plant Biotechnol J 17(12):2228–2230. https://doi.org/10.1111/pbi.13193
Schillberg S, Emans N, Fischer R (2002) Antibody molecular farming in plants and plant cells. Phytochem Rev 1(1):45–54. https://doi.org/10.1023/A:1015880218651
Schillberg S, Raven N, Fischer R, Twyman MR, Schiermeyer A (2013) Molecular farming of pharmaceutical proteins using plant suspension cell and tissue cultures. Curr Pharm Des 19(31):5531–5542. https://doi.org/10.2174/1381612811319310008
Schillberg S, Raven N, Spiegel H, Rasche S, Buntru M (2019) Critical Analysis of the Commercial Potential of Plants for the Production of Recombinant Proteins. Front Plant Sci. https://doi.org/10.3389/fpls.2019.00720
Scholthof HB, Scholthof KBG, Jackson AO (1996) Plant virus gene vectors for transient expression of foreign proteins in plants. Annu Rev Phytopathol 34(1):299–323. https://doi.org/10.1146/annurev.phyto.34.1.299
Sedaghati B, Haddad R, Bandehpour M (2020) Transient expression of human serum albumin (HSA) in tobacco leaves. Mol Biol Rep 47(9):7169–7177. https://doi.org/10.1007/s11033-020-05640-y
Shanmugaraj B, Christine JIB, Phoolcharoen W (2020) Plant molecular farming: a viable platform for recombinant biopharmaceutical production. Plants 9(7):842. https://doi.org/10.3390/plants9070842
Sherman A, Su J, Lin S, Wang X, Herzog RW, Daniell H (2014) Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood 124(10):1659–1668. https://doi.org/10.1182/blood-2013-10-528737
Shim BS, Hong KJ, Maharjan PM, Choe S (2019) Plant factory: new resource for the productivity and diversity of human and veterinary vaccines. Clin Exp Vaccine Res 8:136–139. https://doi.org/10.7774/cevr.2019.8.2.136
Shoji Y, Farrance CE, Bautista J, Bi H, Musiychuk K, Horsey A, Park H, Jaje J, Green BJ, Shamloul M (2012) A plant-based system for rapid production of influenza vaccine antigens. Influenza Other Respir Viruses 6(3):204–210. https://doi.org/10.1111/j.1750-2659.2011.00295.x
Simmons CW, VanderGheynst JS, Upadhyaya SK (2009) A model of Agrobacterium tumefaciens vacuum infiltration into harvested leaf tissue and subsequent in planta transgene transient expression. Biotechnol Bioeng 102(3):965–970. https://doi.org/10.1002/bit.22118
Smith T, O’Kennedy MM, Wandrag DB, Adeyemi M, Abolnik C (2020) Efficacy of a plant-produced virus-like particle vaccine in chickens challenged with Influenza A H6N2 virus. Plant Biotechnol J 18(2):502–512. https://doi.org/10.1111/pbi.13219
So Y, Lee K-J, Kim D-S, Lee J-H, Oh D-B, Hwang K-A, Ko K, Choo Y-K, Ko K (2013) Glycomodification and characterization of anti-colorectal cancer immunotherapeutic monoclonal antibodies in transgenic tobacco. Plant Cell Tissue Organ Cult 113(1):41–49. https://doi.org/10.1007/s11240-012-0249-z
Song S-J, Shin G-I, Noh J, Lee J, Kim D-H, Ryu G, Ahn G, Jeon H, Diao H-P, Park Y, Kim MG, Kim W-Y, Kim Y-J, Sohn E-J, Song CS, Hwang I (2021) Plant-based, adjuvant-free, potent multivalent vaccines for avian influenza virus via Lactococcus surface display. J Integr Plant Biol 63(8):1505–1520. https://doi.org/10.1111/jipb.13141
Stander J, Chabeda A, Rybicki EP, Meyers AE (2021) A plant-produced virus-like particle displaying envelope protein domain III Elicits an immune response against west nile virus in mice. Front Plant Sci. https://doi.org/10.3389/fpls.2021.738619
Stefanova G, Slavov S, Gecheff K, Vlahova M, Atanassov A (2013) Expression of recombinant human lactoferrin in transgenic alfalfa plants. Biol Plant 57(3):457–464
Stoger E, Ma JKC, Fischer R, Christou P (2005) Sowing the seeds of success: pharmaceutical proteins from plants. Curr Opin Biotechnol 16(2):167–173. https://doi.org/10.1016/j.copbio.2005.01.005
Strasser R, Altmann F, Mach L, Glössl J, Steinkellner H (2004) Generation of Arabidopsis thaliana plants with complex N-glycans lacking β1,2-linked xylose and core α1,3-linked fucose. FEBS Lett 561(1–3):132–136. https://doi.org/10.1016/S0014-5793(04)00150-4
Tabayashi N, Matsumura T (2014) Forefront study of plant biotechnology for practical use: development of oral drug for animal derived from transgenic strawberry. Soc Biotechnol J Japn 92:537–539
Tekoah Y, Shulman A, Kizhner T, Ruderfer I, Fux L, Nataf Y, Bartfeld D, Ariel T, Gingis-Velitski S, Hanania U (2015) Large-scale production of pharmaceutical proteins in plant cell culture—the protalix experience. Plant Biotechnol J 13(8):1199–1208. https://doi.org/10.1111/pbi.12428
Thanavala Y, Mahoney M, Pal S, Scott A, Richter L, Natarajan N, Goodwin P, Arntzen CJ, Mason HS (2005) Immunogenicity in humans of an edible vaccine for hepatitis B. Proc Natl Acad Sci USA 102(9):3378–3382. https://doi.org/10.1073/pnas.0409899102
Thomas B, Van Deynze A, Bradford K (2002) Production of therapeutic proteins in plants. UCANR Publications, Oakland, pp 1–12. https://doi.org/10.3733/ucanr.8078
Twyman RM, Stoger E, Schillberg S, Christou P, Fischer R (2003) Molecular farming in plants: host systems and expression technology. Trends Biotechnol 21(12):570–578. https://doi.org/10.1016/j.tibtech.2003.10.002
van Montagu M (2003) Jeff Schell (1935–2003): steering Agrobacterium-mediated plant gene engineering. Trends Plant Sci 8(8):353–354. https://doi.org/10.1016/S1360-1385(03)00160-2
van Roosmalen ML, Kanninga R, El Khattabi M, Neef J, Audouy S, Bosma T, Kuipers A, Post E, Steen A, Kok J (2006) Mucosal vaccine delivery of antigens tightly bound to an adjuvant particle made from food-grade bacteria. Methods 38(2):144–149. https://doi.org/10.1016/j.ymeth.2005.09.015
Verma D, Moghimi B, LoDuca PA, Singh HD, Hoffman BE, Herzog RW, Daniell H (2010) Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci USA 107(15):7101–7106. https://doi.org/10.1073/pnas.0912181107
Vermij P, Waltz E (2006) USDA approves the first plant-based vaccine. Nat Biotechnol 24(3):234. https://doi.org/10.1038/nbt0306-233
von Stackelberg M, Rensing SA, Reski R (2006) Identification of genic moss SSR markers and a comparative analysis of twenty-four algal and plant gene indices reveal species-specific rather than group-specific characteristics of microsatellites. BMC Plant Biol 6(1):1–14. https://doi.org/10.1186/1471-2229-6-9
Vunsh R, Li J, Hanania U, Edelman M, Flaishman M, Perl A, Wisniewski J-P, Freyssinet G (2007) High expression of transgene protein in Spirodela. Plant Cell Rep 26(9):1511–1519. https://doi.org/10.1007/s00299-007-0361-4
Ward BJ, Gobeil P, Séguin A, Atkins J, Boulay I, Charbonneau P-Y, Couture M, D’Aoust M-A, Dhaliwall J, Finkle C, Hager K, Mahmood A, Makarkov A, Cheng MP, Pillet S, Schimke P, St-Martin S, Trépanier S, Landry N (2021) Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat Med 27(6):1071–1078. https://doi.org/10.1038/s41591-021-01370-1
Winicur ZM, Feng Zhang G, Andrew Staehelin L (1998) Auxin deprivation induces synchronous Golgi differentiation in suspension-cultured tobacco BY-2 cells. Plant Physiol 117(2):501–513. https://doi.org/10.1104/pp.117.2.501
Yang M, Sun H, Lai H, Hurtado J, Chen Q (2018) Plant-produced Zika virus envelope protein elicits neutralizing immune responses that correlate with protective immunity against Zika virus in mice. Plant Biotechnol J 16(2):572–580. https://doi.org/10.1111/pbi.12796
Yusibov V, Rabindran S (2008) Recent progress in the development of plant derived vaccines. Expert Rev Vaccines 7(8):1173–1183. https://doi.org/10.1586/14760584.7.8.1173
Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M (2012) Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Proc Natl Acad Sci USA 109(12):E690–E697. https://doi.org/10.1073/pnas.1115485109
Zhao H, Tan Z, Wen X, Wang Y (2017) An improved syringe agroinfiltration protocol to enhance transformation efficiency by combinative use of 5-azacytidine, ascorbate acid and Tween-20. Plants 6(1):9. https://doi.org/10.3390/plants6010009
Zhou J-Y, Cheng L-Q, Zheng X-J, Wu J-X, Shang S-B, Wang J-Y, Chen J-G (2004) Generation of the transgenic potato expressing full-length spike protein of infectious bronchitis virus. J Biotechnol 111(2):121–130. https://doi.org/10.1016/j.jbiotec.2004.03.012
Acknowledgements
This research was supported by a research grant from the Rural Development Administration project (Project No. PJ01570102, JL), Republic of Korea.
Author information
Authors and Affiliations
Contributions
JL and KRL wrote and reviewed the manuscript. Three types of PMPs and tools and techniques for PMP expression were reviewed by SKL. PMP expression platforms were reviewed by JSP. Final compilation and editing were done by JL and KRL. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, J., Lee, SK., Park, JS. et al. Plant-made pharmaceuticals: exploring studies for the production of recombinant protein in plants and assessing challenges ahead. Plant Biotechnol Rep 17, 53–65 (2023). https://doi.org/10.1007/s11816-023-00821-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11816-023-00821-0