Abstract
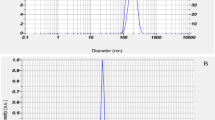
To improve the bioavailability of ampicillin trihydrate (AMP) as a poorly water-soluble drug, the nanonization of AMP particles was carried out by solvent anti-solvent precipitation for the first time. In this method the subcritical water (SW) and cold water at ambient conditions were utilized as the solvent and anti-solvent, respectively. At first, the solubility of AMP in SW was measured. The solubility of AMP in SW at a constant pressure of 5 MPa and the temperature range from 303.15 to 403.15 K was found to range from 0.380×10−3 to 17.689×10−3 mole fractions. The effects of three independent variables, including SW temperature, polyethylene glycol concentration, and anti-solvent temperature, on the particle size and morphology of the precipitated nanoparticles were studied. The obtained results of analyses confirmed that the AMP particles were nanosized to the smallest mean size of 66.5 nm using an environmentally friendly method without the requirement of organic solvents and related post-processing purification stages.
Similar content being viewed by others
Abbreviations
- AMP:
-
ampicillin trihydrate
- SW:
-
subcritical water
- SAS:
-
supercritical anti-solvent
- SAA:
-
supercritical-assisted atomization
- GAS:
-
supercritical gas anti-solvent
- DMSO:
-
dimethyl sulfoxide
- BBD:
-
Box-Behnken design
- FTIR:
-
Fourier transform infrared spectroscopy
- XRD:
-
X-ray diffraction
- SEM:
-
scanning electron microscopy
- DLS:
-
dynamic light scattering
- PEG:
-
polyethylene glycol
- x2 :
-
equilibrium mole fraction
- T:
-
temperature [K]
- RSM:
-
response surface methodology
- R2 :
-
coefficient of determination
References
G. Absalan, A. Abbaspour, M. Jafari, M. Nekoeinia and H. Ershadifar, J Iran Chem. Soc., 12, 879 (2015).
A. Tenorio, M. D. Gordillo, C. Pereyra and E. J. Martinez de la Ossa, J. Supercrit. Fluids, 40, 308 (2007).
E. Reverchon, G. Della Porta and A. Spada, J. Pharm. Pharmacol., 55, 1465 (2003).
S. K. Sharma, L. Singh and S. Singh, Sch. J. Appl. Med. Sci., 1, 291 (2013).
Y. Fan, A. C. Pauer, A. A. Gonzales and H. Fenniri, Int. J. Nanomedicine, 14, 7281 (2019).
J. W. Poole and C. K. Bahal, J. Pharm. Sci., 57, 1945 (1968).
Y. Wu, A. Loper, E. Landis, L. Hettrick, L. Novak, K. Lynn, C. Chen, K. Thompson, R. Higgins and U. Batra, Int. J. Pharm., 285, 135 (2004).
P. Khadka, J. Ro, H. Kim, I. Kim, J. T. Kim, H. Kim, J. M. Cho, G. Yun and J. Lee, Asian J. Pharm. Sci., 9, 304 (2014).
Y. Pu, J. X. Wang, D. Wang, N. R. Foster and J. F. Chen, Chem. Eng. Process, 140, 36 (2019).
G. Sodeifian, F. Razmimanesh and S. A. Sajadian, J. Mol. Liq., 297, 11740 (2020).
G. Sodeifian, S. A. Sajadian, N. S. Ardestani and F. Razmimanesh, J. Supercrit. Fluids, 147, 241 (2019).
Z. Sayyar and H. Jafarizadeh-Malmiri, Chem. Eng. Process, 153, 107938 (2020).
Q. Can, J. Carlfors and C. Turner, Chin. J Chem. Eng., 17, 344 (2009).
O. Kayser, A. Lemke and N. Hernandez-Trejo, Curr. Pharm. Biotechnol., 6, 3 (2005).
C. Leuner and J. Dressman, Eur. J. Pharm. Biopharm., 50, 47 (2000).
S. Stolnik, L. Illum and S. Davis, Adv. Drug Deliv. Rev., 16, 195 (1995).
C. M. Keck and R. H. Muller, Eur. J. Pharm. Biopharm., 62, 3 (2006).
M. D. Louey, M. V. Oort and A. J. Hickey, Pharm. Res., 21, 1200 (2004).
N. Rasenack, H. Steckel and B. W. Muller, J. Pharm. Sci., 92, 35 (2003).
K. H. Song, C. H. Lee, J. S. Lim and Y. W. Lee, Korean J. Chem. Eng., 19, 139 (2002).
C. Chinnarasu, A. Montes, C. Pereyra, L. Casas, M. Teresa, C. Mantell, S. Pattabhi and E. Ossa, Korean J. Chem. Eng., 33, 594 (2016).
S. J. Park and S. D. Yeo, Korean J. Chem. Eng., 25, 575 (2008).
C. K. Kim, B. C. Lee, Y. W. Lee and H. Kim, Korean J. Chem. Eng., 26, 1125 (2009).
S. V. Dalvi and M. Mukhopadhyay, Powder Technol., 195, 190 (2009).
F. Masoodiyeh, J. Karimi-Sabet, A. R. Khanchi and M. R. Mozdianfard, Powder Technol., 269, 461 (2015).
A. Montes, A. I. Tenorio, M. D. Gordillo, C. M. Pereyra and E. J. Martinez, Ind. Eng. Chem. Res., 50, 2343 (2011).
N. Esfandiari and S. M. Ghoreishi, AAPS Pharm. Sci. Tech., 6, 1263 (2015).
M. Khajenoori, A. Haghighi Asl and F. Hormozi, Chin. J. Chem. Eng., 17, 359 (2009).
O. Ahmadi and H. Jafarizadeh-Malmiri, Food Sci. Biotechnol., 29, 783 (2020).
R. Alenezi, G. A. Leeke, R. C. D. Santos and A. R. Khan, Chem. Eng. Res. Des., 87, 867 (2009).
R. J. Fernandez-Prini, H. R. Corti and M. L. Japas, High-temperature aqueous solutions: Thermodynamic properties, CRC Press, Boca Raton (1992).
J. N. Park, A. Alinehari, H. C. Woo and B. S. Chun, Korean J. Chem. Eng., 29, 1604 (2012).
A. G. Carr, R. Mammucari and N. R. Foster, Ind. Eng. Chem. Res., 49, 3403 (2010).
H. Share Mohammadi, A. Haghighi Asl and M. Khajenoori, Chin. J. Chem. Eng., 8, 2620 (2020).
X. Y. Chen, Y. L. Shang, Y. H. Li, J. X. Wang, A. G. Maimouna, Y. X. Li, D. Zou, N. R. Foster, J. Yun and Y. Pu, Chem. Eng. J., 263, 20 (2015).
E. D. Hugger, B. L. Novak, P. S. Burton, K. L. Audus and R. T. Borchardt, J. Pharm. Sci., 91, 1991 (2002).
M. Rahimi, P. Valeh-e-Sheyda and H. Rashidi, Korean J. Chem. Eng., 34, 3017 (2017).
A. G. Carr, R. Mammucari and N. R. Foster, Ind. Eng. Chem. Res., 49, 9385 (2010).
Y. Pu, J. Lu, D. Wang, F. Cai, J. Wang, N. R. Foster and J. F. Chen, Powder Technol., 321, 197 (2017).
A. G. Carr, R. Mammucari and N. R. Foster, Chem. Eng. J., 172, 1 (2011).
B. Kayan, Y. Yang, E. J. Lindquist and A. M. Gizir, J. Chem. Eng. Data, 55, 2229 (2010).
B. Kapalavavi, J. Ankney, M. Baucom and Y. Yang, J. Chem. Eng. Data, 59, 912 (2014).
M. Uematsu and E. U. Frank, J. Phys. Chem. Ref. Data, 9, 1291 (1980).
E. R. Caffarena and J. R. Grigera, Physica A Stat. Mech. Appl., 342, 34 (2004).
Y. Takebayashi, K. Sue, S. Yoda, Y. Hakuta and T. Furuya, J. Chem. Eng. Data, 57, 1810 (2012).
K. Shinoda, J. Phys. Chem. A, 81, 1300 (1977).
D. J. Miller and S. B. Hawthorne, Anal. Chem., 70, 1618 (1998).
S. Akay, B. Kayan, D. Cunbin, J. Wang and Y. Yang, J. Mol. Liq., 253, 270 (2018).
S. Karthika, T. Radhakrishnan and P. Kalaichelvi, Cryst. Growth Des., 16, 6663 (2016).
M. Turk, J. Supercrit. Fluids, 18, 169 (2000).
G. Sodeifian, S. A. Sajadian and S. Daneshyan, J. Supercrit. Fluids, 140, 72 (2018).
Y. Pu, Y. Li, D. Wang, N. R. Foster, J. X. Wang and J. F. Chen, Powder Technol., 308, 200 (2017).
Y. Pu, X. Wen, Y. Li, D. Wang, N. R. Foster and J. F. Chen, Powder Technol., 305, 125 (2017).
A. G. Carr, R. Mammucari and N. R. Foster, Int. J. Pharm., 405, 169 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammadi, H.S., Asl, A.H. & Khajenoori, M. Solubility measurement and preparation of nanoparticles of ampicillin using subcritical water precipitation method. Korean J. Chem. Eng. 38, 2304–2312 (2021). https://doi.org/10.1007/s11814-021-0891-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-021-0891-4