Abstract
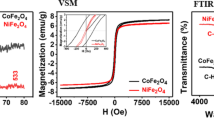
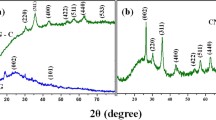
Magnetic cobalt ferrites (CoFe2O4) were synthesized by sol-gel method. These nanoparticles were ultra-sonicated with surface modified multi-walled carbon nanotubes (SM-MWCNTs) to form CoFe2O4/SM-MWCNTs nanocomposites. The as-prepared materials were used as an adsorbent for the removal of hexavalent chromium (Cr(VI)) arising from the presence of dichromate ions (Cr2O2−7 ) in the electroplating effluent. The synthesized nanocomposites were characterized by field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), X-Ray diffraction (XRD), Fourier transmission infrared spectroscopy (FT-IR), raman spectroscopy, thermo-gravimetric analysis (TGA), and zeta analyzer. The effect of the environmental chemistry of the solution on the adsorption has been discussed. The adsorption isotherm of Cr(VI) adsorption onto the as-synthesized CoFe2O4/SM-MWCNTs best fitted the Langmuir Adsorption Isotherm model. The high adsorption capacity of 100mg/g was achieved at 40°C under optimized conditions. Besides, the magnetic properties of synthesized CoFe2O4/SM-MWCNTs nanocomposites allow them to separate from the aqueous solution by magnetization easily. Even after seven consecutive adsorption-desorption cycles, the CoFe2O4/SM-MWCNTs nanocomposites presented an efficiency loss of less than 20% for the removal of Cr(VI) ions. This study clearly shows that cobalt nanocomposites are promising candidates in environmental applications.
Similar content being viewed by others
References
J. Xu, Z. Cao, Y. Zhang, Z. Yuan, Z. Lou, X. Xu and X. Wang, Chemosphere, 195, 351 (2018).
S. Iijima, Nature, 354, 56 (1991).
H. Chang and H. Wu, Energy Environ. Sci., 6(12), 3483 (2013).
R. Sepahvand and R. Mohamadzade, J. Sci. Islam. Repub. Iran, 22(2), 177 (2011).
V. Georgakilas, D. Gournis, V. Tzitzios, L. Pasquato, M. Guldi and M. Prato, J. Mater. Chem., 17, 2679 (2007).
H. Zheng, J. Kreisel, Y. Chu, R. Ramesh and L. Salamanca-riba, Appl. Phys. Lett., 90, 113113 (2007).
T. N. Diva, K. Zare, F. Taleshi and M. Yousefi, J. Nanostructure Chem., 7, 273 (2017).
M. Hadi, M. Mohammad, A. Kumar and B. Heibati, Chem. Eng. J., 279, 344 (2015).
M. S. Gaikwad and C. Balomajumder, J. Environ. Chem. Eng., 5(1), 45 (2017).
J. O. M. Neto, C. R. Bellato and D. C. Silva, Chemosphere, 218, 391 (2019).
W. Chen, Z. Lu, B. Xiao, P. Gu, W. Yao, J. Xing, A. M. Asiri, K. A. Alamry, X. Wang and S. Wang, J. Clean. Prod., 211, 1250 (2019).
P. A. M. Mourão, P. J. M. Carrott and M. M. L. Ribeiro Carrott, Carbon, 44(12), 2422 (2006).
N. Li, M. Zheng, X. Chang, G. Ji, H. Lu, L. Xue, L. Pan and J. Cao, J. Solid State Chem., 184, 953 (2011).
T. Zhao, X. Ji, X. Guo, W. Jin, A. Dang, H. Li and T. Li, Chem. Phys. Lett., 653, 202 (2016).
C. Luo, Z. Tian, B. Yang, L. Zhang and S. Yan, Chem. Eng. J., 234, 266 (2013).
J. Hu, C. Chen, X. Zhu and X. Wang, J. Hazard. Mater., 162(2), 1542 (2009).
L. Tang, G. D. Yang, G. M. Zeng, Y. Cai, S. S. Li, Y Y. Zhou, Y. Pang, Y. Y. Liu, Y. Zhang and B. Luna, Chem. Eng. J., 239, 114 (2014).
J. Hu, G. Chen and I. M. C. Lo, Water Res., 39(18), 4528 (2005).
M. H. Dehghani, M. M. Taher, A. K. Bajpai, B. Heibati, I. Tyagi, M. Asif, S. Agarwal and V. K. Gupta, Chem. Eng. J., 279, 344 (2015).
T. Wajima, Y. Umeta, S. Narita and K. Sugawara, DES, 249(1), 323 (2009).
M. E. Argun, S. Dursun, C. Ozdemir and M. Karatas, J. Hazard. Mater., 141, 77 (2007).
Y.-H. Li, S. Wang, J. Wei, X. Zhang, C. Xu, Z. Luan, D. Wu and B. Wei, Chem. Phys. Lett., 357, 263 (2002).
A. Bhatnagar, E. Kumar and M. Sillanpää, Chem. Eng. J., 163(3), 317 (2010).
M. L. Paul, J. Samuel, S. B. Das, S. Swaroop, N. Chandrasekaran and A. Mukherjee, Ind. Eng. Chem. Res., 51, 46 (2012).
R. Milena, H. Buchtov and P. Jano, Water Res., 37, 4938 (2003).
X. Yang, Y. Wan, Y. Zheng, F. He, Z. Yu, J. Huang, H. Wang, Y. S. Ok, Y. Jiang and B. Gao, Chem. Eng. J., 366, 608 (2019).
S. Chen, Q. Yue, B. Gao and X. Xu, J. Colloid Interface Sci., 349(1), 256 (2010).
C. Cabrera, C. Gabaldón and P. Marzal, J. Chem. Technol. Biotechnol., 80(4), 477 (2005).
P. Wang, M. Du, H. Zhu, S. Bao, T. Yang and M. Zou, J. Hazard. Mater., 286, 533 (2015).
J. Huang, Y. Cao, Q. Shao, X. Peng and Z. Guo, Ind. Eng. Chem. Res., 56(38), 10689 (2017).
I. Enniya, L. Rghioui and A. Jourani, Sustain. Chem. Pharm., 7, 9 (2018).
S. Rajput, C. U. Pittman and D. Mohan, J. Colloid Interface Sci., 468, 334 (2016).
C. G. Lee, S. Lee, J. A. Park, C. Park, S. J. Lee, S. B. Kim, B. An, S. T. Yun, S. H. Lee and J. W. Choi, Chemosphere, 166, 203 (2017).
P. B. Vilela, A. Dalalibera, E. C. Duminelli, V. A. Becegato and A. T. Paulino, Environ. Sci. Pollut. Res., 26(28), 28481 (2019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supporting Information
Additional information as noted in the text. This information is available via the Internet at http://www.springer.com/chemistry/journal/11814.
Supporting Information
11814_2020_516_MOESM1_ESM.pdf
Fabrication of magnetic cobalt ferrite nanocomposites: an advanced method of removal of toxic dichromate ions from electroplating wastewater
Rights and permissions
About this article
Cite this article
Verma, B., Balomajumder, C. Fabrication of magnetic cobalt ferrite nanocomposites: an advanced method of removal of toxic dichromate ions from electroplating wastewater. Korean J. Chem. Eng. 37, 1157–1165 (2020). https://doi.org/10.1007/s11814-020-0516-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-020-0516-3