Abstract
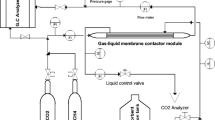
The chemical absorption of CO2 in a monoethanolamine (MEA) solution by a ceramic hollow fiber membrane contactor (HFMC) was investigated experimentally and numerically to obtain the best compromise between the mass transfer coefficient and structural characteristics such as membrane pore size and porosity. The mathematical model derived is based on the three resistances in the resistance-in-series model. The accuracy of the numerical simulation was verified quantitatively by the experimental data obtained in this study. A good agreement between experimental and computational results was found with an average absolute deviation (AAD) between observed data and predicted values of 2.86%. In addition, the effects of the operating condition (i.e., gas and liquid flow rates) on the mass transfer coefficients for ceramic HFMC systems were also studied, revealing that the membrane and gas-phase mass transfer resistances were dominant factors in the overall mass transfer. In conclusion, the present study suggests that the membrane structure plays a very important role in the optimization of HFMC performance. In fact, the best results were obtained with an intermediate range of the pore size between 102 and 104 nm, corresponding to the best compromise between performance (i.e., overall mass transfer coefficient) and applicability (i.e., breakthrough pressure).
Similar content being viewed by others
Abbreviations
- A:
-
cross section area [m2]
- B:
-
Boltzmann’s constant
- \({{\rm{C}}_{C{O_2}}},\;{\rm{C}}_{C{O_2}}^*\) :
-
CO2 concentration in solvent [mol/m2]
- \(C_{CO_2,MEA}\) :
-
concentration of CO2 in MEA solution [mol/m3]
- C MEA :
-
molarity of MEA solution [mol/m3]
- de :
-
hydraulic diameter [m]
- di, do, dlm :
-
hollow fiber outer, inner and log mean diameters [m]
- dp :
-
membrane pore diameter [nm]
- \({{\rm{D}}_{C{O_2},\;gas}}\) :
-
bulk diffusion coefficient [m2/s]
- \({{\rm{D}}_{C{O_2},\;{H_2}O}}\) :
-
diffusion coefficient of CO2 in MEA solution [m2/s]
- \({{\rm{D}}_{C{O_2},\;m}}\) :
-
effective membrane diffusion coefficient [m2/s]
- \({{\rm{D}}_{C{O_2},\;MEA}}\) :
-
diffusion coefficient of CO2 in the MEA solution [m2/s]
- DKn :
-
Knudsen diffusion coefficient [m2/s]
- DMEA :
-
diffusion coefficient of MEA in aqueous MEA solution [m2/s]
- \({{\rm{D}}_{{N_2}O,\;{H_2}O}}\) :
-
diffusion coefficient of N2O in MEA solution [m2/s]
- \({{\rm{D}}_{{N_2}O,\;MEA}}\) :
-
diffusion coefficient of N2O in MEA solution [m2/s]
- E:
-
enhancement factor
- Exp:
-
experimental data
- E∞ :
-
infinite enhancement factor
- Gz:
-
Graetz number
- H:
-
Henry’s law constant [Pa·m3/mol]
- Ha:
-
Hatta number
- \({{\rm{J}}_{C{O_2}}}\) :
-
mass transfer flux [mol/m2·s−1]
- k:
-
reaction rate constant of CO2-MEA reaction [m3/mol·s]
- kg, km, kl :
-
mass transfer coefficient in gas, membrane, and liquid phase [m/s]
- K L :
-
overall mass transfer coefficient [m/s]
- \({{\rm{M}}_{C{O_2}}}\), \({{\rm{M}}_{{N_2}}}\) :
-
molecular weight of CO2 and N2
- L:
-
fiber length [m]
- \({{\rm{p}}_{C{O_2},\,g}}\) :
-
partial pressure of the CO2 in the gas phase [Pa]
- P:
-
pressure [Pa]
- ΔP:
-
breakthrough pressure [Pa]
- rp :
-
membrane pore radius [m]
- Re:
-
Reynolds number
- Sc:
-
Schmidt number
- Sh:
-
Sherwood number
- Sim:
-
Simulated data
- T:
-
temperature [K]
- T*:
-
dimensionless temperature
- U:
-
wetted perimeter [m]
- vi :
-
liquid velocity [m/s]
- vR :
-
stoichiometric coefficient
- \({{\rm{x}}_{{H_2}O}},\;{{\rm{x}}_{MEA}}\) :
-
mole fraction of H2O and MEA in the solution
- γ :
-
surface tension
- δ :
-
membrane thickness [m]
- ε :
-
membrane porosity
- \({\varepsilon _{C{O_2}}},\;{\varepsilon _{{N_2}}}\) :
-
characteristic energy of CO2 and N2
- θ :
-
contact angle
- μ :
-
gas viscosity [kg/m·s]
- ρ :
-
gas density [kg/m3]
- \({\sigma _{C{O_2}}},\;{\sigma _{{N_2}}}\) :
-
characteristic length of CO2 and N2 [Å]
- τ m :
-
tortuosity
- ∅:
-
packing density
- Ω D :
-
diffusion collision integral
References
Y. Kim, J. Ryu and I. Lee, Korean Chem. Eng. Res., 47(5), 531 (2009).
G. Bakeri, M. Rezaei-DashtArzhandi, A. F. Ismail, T. Matsuura, M. S. Abdullah and N. B. Cheer, Korean J. Chem. Eng., 34(1), 160 (2017).
N. Nabian, A. A. Ghoreyshi, A. Rahimpour and M. Shakeri, Korean J. Chem. Eng., 32(11), 2204 (2015).
D. Jeong, M. Yun, J. Oh, I. Yum and Y. Lee, Korean J. Chem. Eng., 27(3), 939 (2010).
B. Ozturk and R. Hughes, Chem. Eng. J., 195-196, 122 (2012).
S. Boributh, W. Rongwong, S. Assabumrungrat, N. Laosiripojana and R. Jiraratananon, J. Membr. Sci., 401-402, 175 (2012).
S. A. Hashemifard, T. Matsuura, A. F. Ismail, M. R. D. Arzhandi, D. Rana and G. Bakeri, Chem. Eng. J., 281, 970 (2015).
K. Li, J. Kong and X. Tan, Chem. Eng. Sci., 55, 5579 (2000).
S. Atchariyawut, C. Feng, R. Wang, R. Jiraratananon and D. T. Liang, J. Membr. Sci., 285, 272 (2006).
G. Bakeri, A. F. Ismail, D. Rana and T. Matsuura, Chem. Eng. J., 198-199, 327 (2012).
F. Korminouri, M. Rahbari-Sisakht, T. Matsuura and A. F. Ismail, Chem. Eng. J., 264, 453 (2015).
L. Wang, Z. Zhang, B. Zhao, H. Zhang, X. Lu and Q. Yang, Sep. Purif. Technol., 116, 300 (2013).
H. J. Lee, E. Magnone and J. H. Park, J. Membr. Sci., 494, 143 (2015).
S. Koonaphapdeelert, Z. Wu and K. Li, Chem. Eng. Sci., 64, 1 (2009).
H. J. Lee and J. H. Park, J. Membr. Sci., 518, 79 (2016).
M. C. Yang and E. L. Cussler, AIChE J., 32, 1910 (1986).
H. Kreulen, C. A. Smolders, G. F. Versteeg and W. P. M. van Swaaij, J. Membr. Sci., 78, 197 (1993).
G. F. Versteeg and W. P. M. van Swaalj, J. Chem. Eng. Data, 33, 29 (1988).
J. J. Ko, T. C. Tsai, C. Y. Lin, H. M. Wang and M. H. Li, J. Chem. Eng. Data, 46, 160 (2000).
A. Gabelman and S.-T. Hwang, J. Membr. Sci., 159, 61 (1999).
J. L. Li and B. H. Chen, Sep. Purif. Technol., 41, 109 (2005).
S. Boributh, S. Assabumrungrat, N. Laosiripojana and R. Jiraratananon, J. Membr. Sci., 380, 21 (2011).
B. E. Poling, J. M. Prausnitz and J. P. O’connell, Properties of gases and liquids, fifth Ed., McGraw-Hill (2004).
M. C. Yang and E. L. Cussler, AIChE J., 32, 1910 (1986).
M. J. Costello, A. G. Fane, P. A. Hogan and R. W. Schofield, J. Membr. Sci., 80, 1 (1993).
P. Côté, J. L. Bersillon and A. Huyard, J. Membr. Sci., 47, 91 (1989).
R. Prasad and K. K. Sirkar, AIChE J., 34, 177 (1988).
W. J. DeCoursey, Chem. Eng. Sci., 29, 1867 (1974).
P. M. M. Blauwhoff, G. F. Versteef and W. P. M. van Swaaij, Chem. Eng. Sci., 38, 1411 (1983).
E. D. Snijder, M. J. M. te Riele, G. F. Versteeg and W. P. M. van Swaaij, J. Chem. Eng. Data, 38, 475 (1993).
S. Khaisri, D. deMontigny, P. Tontiwachwuthikul and R. Jiraratananon, J. Membr. Sci., 347, 228 (2010).
A. Penttilä, C. Dell’Era, P. Uusi-Kyyny and V. Alopaeus, Fluid Phase Equilib., 311, 59 (2011).
V. Y. Dindore, D. W. F. Brilman, F. H. Geuzebroek and G. F. Versteeg, Sep. Purif. Technol., 40, 133 (2004).
S. A. Jayarathna, A. Weerasooriya, S. Dayarathna, D. A. Eimer and M. C. Melaaen, J. Chem. Eng. Data, 58, 986 (2013).
M. Calderer, I. Jubany, R. Pérez, V. Martí and J. de Pablo, Chem. Eng. J., 165, 2 (2010).
J. H. Kim, S. K. Hong and S. J. Park, Korean Chem. Eng. Res., 45, 619 (2007).
E. Quijada-Maldonado, T. A. M. Aelmans, G. W. Meindersma and A. B. de Haan, Chem. Eng. J., 223, 287 (2013).
A. Aboudheir, P. Tontiwachwuthikul, A. Chakma and R. Idem, Chem. Eng. Sci., 58, 5195 (2003).
W. Rongwong, R. Jiraratananon, and S. Atchariyawut, Sep. Purif. Technol., 69, 118 (2009).
Y. Kim and S. Yang, Sep. Purif. Technol., 21, 101 (2000).
Y. Lv, X. Yu, S.-T. Tu, J. Yan and E. Dahlquist, Appl. Energy, 112, 755 (2013).
Y. Lv, X. Yu, J. Jia, S.-T. Tu, J. Yan and E. Dahlquist, Appl. Energy, 90(1), 167 (2012).
S. Yan, M. Fang, W. Zhang, S. Wang, Z. Xu, Z. Luo and K. Cen, Fuel Process. Technol., 88, 501 (2007).
W. Rongwong, S. Assabumrungrat and R. Jiraratananon, J. Membr. Sci., 429, 396 (2013).
S. Atchariyawut, R. Jiraratananon and R. Wang, J. Membr. Sci., 304, 163 (2007).
R. Faiz and M. Al-Marzouqi, J. Membr. Sci., 342, 269 (2009).
M. R. DashtArzhandi, A. F. Ismail, T. Matsuura, B. C. Ng and M. S. Abdullah, Chem. Eng. J., 269, 51 (2015).
M. Rahbari-Sisakht, D. Rana, T. Matsuura, D. Emadzadeh, M. Padaki and A. F. Ismail, Chem. Eng. J., 246, 306 (2014).
A. Hasanoğlu, J. Romero, B. Pérez and A. Plaza, Chem. Eng. J., 160, 530 (2010).
A. Mansourizadeh and A. F. Ismail, Chem. Eng. J., 165, 980 (2010).
Acknowledgements
This work was supported by a Korea CCS R&D Center (KCRC) grant funded by the Korean government (Ministry of Science, ICT & Future Planning) (No. 2014M1A8A1049314).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H.J., Binns, M., Park, S.J. et al. An experiment and model of ceramic (alumina) hollow fiber membrane contactors for chemical absorption of CO2 in aqueous monoethanolamine (MEA) solutions. Korean J. Chem. Eng. 36, 1669–1679 (2019). https://doi.org/10.1007/s11814-019-0351-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-019-0351-6