Abstract
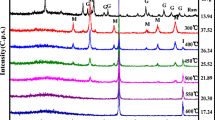
Ferric hydroxide adsorbent was prepared by a chemical treatment process with H2O2, NaOH, and aeration from a Fe2(SO4)3 aqueous solution as a side product discharged from the hydrometallurgical process used to extract neodymium. The ferric hydroxide was used as an adsorbent to prevent eutrophication in water. At the time of synthesis, the most important process variable is the pH condition, which, in this experiment, was changed from pH 3 to 13. The cost of synthesizing ferric hydroxide was sharply reduced by using ferric sulfate, which is considered a side product of the aforementioned hydrometallurgical process, as a starting material, and an adsorbent with high adsorption ability was prepared by controlling the pH level. Microstructural characterization of the synthesized ferric hydroxide revealed particles with a specific surface area of 194.2 m2/g and an average pore diameter of 2.66 nm at pH 6 and 298 K. A column-type packed-bed adsorption experiment was conducted under the following conditions: a flow rate of 0.567 BV/min (3.2 mL/min), 298 K, and atmospheric pressure. The results of the adsorption performance test indicated that the adsorption efficiency of phosphate at concentrations of 10 ppm was 100% at a flow rate of 0.567 BV/min within a contact time of 2 min, and the maximum adsorption capacity for phosphate ions was 65 mg/g.
Similar content being viewed by others
References
J. Huang, C. Xu, B. G. Ridoutt, X. Wang and P. Ren, J. Cleaner Production, 159, 171 (2017).
M. West, N. Fenner, R. Gough and C. Freeman, Ecological Engineering (2017), DOI:10. 1016/j.ecoleng.2017.07.033.
F. Xie, F. Wu, G. Liu, Y. Mu, C. Feng, H. Wang and J. P. Giesy, Environ. Sci. Technol., 48, 582 (2014).
Q. Liu, P. Hu, J. Wang, L. Zhang and R. Huang, J. Taiwan Institute of Chemical Engineers, 59, 311 (2016).
M. Arshadi, S. Foroughifard, J. E. Gholtash and A. Abbaspourrad, J. Colloid Interface Sci., 452, 69 (2015).
G. Li, D. Chen, W. Zhao and X. Zhang, J. Environ. Chem. Eng., 3, 515 (2015).
M. E. Bouraie and A. A. Masoud, Appl. Clay Sci., 140, 157 (2017).
W. Xiong, J. Tong, Z. Yang, G. Zeng, Y. Zhou, D. Wang, P. Song, R. Xu, C. Zhang and M. Cheng, J. Colloid Interface Sci., 493, 17 (2017).
D. Mitrogiannis, M. Psychoyou, I. Baziotis, V. J. Inglezakis, N. Koukouzas, N. Tsoukalas, D. Palles, E. Kamitsos, G. Oikonomou and G. Markou, Chem. Eng. J., 320, 510 (2017).
C. Zhang, Y. Li, F. Wsng, Z. Yu, J. Wei, Z. Yang, C. Ma, Z. Li, Z. Xu and G. Zeng, Appl. Surface Sci., 396, 1783 (2017).
J. Dai, H. Yan, Y. Shangguan, Q. Zheng and R. Cheng, Chem. Eng. J., 166, 970 (2011).
Z. Ren, L. Shao and G. Zhang, Water Air Soil Pollut., 223, 4221 (2012).
J.-W. Choi, K.-S. Kwon, S. Lee, B. An, S.-W. Hong and S.-H. Lee, Water Air Soil Pollut., 225, 1835 (2014).
K. Wu, T. Liu, C. Ma, B. Chang, R. Chen and X. Wang, Environ. Sci. Pollut. Res., 21, 620 (2014).
S. H. Lee, S. W. Hing and J. W. Choi, KIC News, 14, 22 (2011).
B. Beverskog and I. Puigdomenech, Corrosion Science, 38, 2121 (1996).
P. Wilfert, P. S. Kumar, L. Korving, G.-J. Witkamp and M. C. M. van Loosdrecht, Environ. Sci. Technol., 49, 9400 (2015).
E. M. Michalos and A. A. Rouff, Spectroscopy Lett., 48, 695 (2015).
A. Munoz, F. M. Torres, J. M. Estela and V. Cerda, Anal. Chim. Acta, 350, 21 (1997).
J. R. Regalbuto, A. Navada, S. Shadid, M. L. Bricker and Q. Chen, J. Catal., 184, 335 (1999).
W. A. Spieker and J. R. Regalbuto, Chem. Eng. Sci., 56, 3491 (2001).
X. Hao, W. A. Spieker and J. R. Regalbuto, J. Colloid Interface Sci., 267, 259 (2003).
K. B. Agashe and J. R. Regalbuto, J. Colloid Interface Sci., 185, 174 (1997).
W. A. Spieker, J. Liu, J. T. Miller, A. J. Kropf and J. R. Regalbuto, Appl. Catal. A: Gen., 232, 219 (2002).
W. A. Spieker, J. Liu, X. Hao, J. T. Miller, A. J. Kropf and J. R. Regalbuto, Appl. Catal. A: Gen., 243, 53 (2003).
J. S. Noh and J. A. Schwarz, J. Colloid Interface Sci., 130, 157 (1988).
J. R. Regalbuto, O. Ansel and J. T. Miller, Topics in Catalysis, 39, 237 (2006).
J. Park and J. R. Regalbuto, J. Colloid Interface Sci., 175, 239 (1995).
R. O. James and T. W. Healy, J. Colloid Interface Sci., 40, 65 (1972).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, HS., Chung, K.W., Kim, CJ. et al. Characteristics of phosphate adsorption on ferric hydroxide synthesized from a Fe2(SO4)3 aqueous solution discharged from a hydrometallurgical process. Korean J. Chem. Eng. 35, 470–478 (2018). https://doi.org/10.1007/s11814-017-0287-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-017-0287-7