Abstract
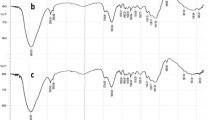
Syzygium cumini L. leaf powder and Cd(II) loaded samples were characterized using FTIR and SEM techniques. The biosorption of cadmium ions from aqueous solution was studied in a batch adsorption system as a function of pH, contact time, adsorbate, adsorbent, anion and cation concentrations. The biosorption capacities and rates of transfer of cadmium ions onto S. cumini L. were evaluated. The kinetics could be best described by both linear and nonlinear pseudo-second order models. The isothermic data fitted to various models in the order Freundlich>Redlich-Peterson>Langmuir>Temkin. The maximum adsorption capacity of S. cumini L. leaves at room temperature was estimated to be 34.54 mg g−1. The negative values of ΔG0 indicated the feasibility of the adsorption process. The endothermic nature was confirmed by the positive value of the enthalpy change (ΔH0=3.7 kJ mol−1). The positive value of entropy change (ΔS0=16.87 J mol−1 K−1) depicted internal structural changes during the adsorption process.
Similar content being viewed by others
References
B. Thomson and W. Turney, Wat. Environ. Res., 67, 527 (1995).
K. Seki, N. Saito and M. Aoyama, Wood Sci. Technol., 31, 441 (1997).
A. Denizli, E. Buyuktuncel, O. Genc and E. Piskin, Anal. Lett., 31, 2791 (1998).
L. Friberg, G. F. Nordberg and B. Vouk (Eds.), Handbook on the Toxicology of Metals, Elsevier/Biomedical Press, North-Holland/Amsterdam (1979).
J.W. Patterson and R. Passino, Metal speciation separation and recovery, Lewis Publishers, Chelsea, MI, USA (1987).
E. Lehoczky, L. Szabo, Sz. Horvath, P. Marth and I. Szabados, Commun. Soil Sci. Plant Anal., 29, 1903 (1998).
T. Kjellstrom, K. Shiroishi and P. E. Erwin, Environ. Res., 98, 318 (1977).
MINAS Pollution Control Acts, Rules, Notification issued there under Central Pollution Control Board, Ministry of Environment and Forests, Government of India, New Delhi, September (2001).
R.A. Beauvais and S. F. Alexandratos, React. Funct. Polym., 36, 113 (1998).
N. Kuyucak, in: Biosorption of heavy metals, B. Volesky (Ed.), CRC Press, Boca Raton (1990).
P. King, N. Rakesh, S. Beenalahari, Y. P. Kumar and V. S. R. K. Prasad, J. Hazard. Mater., 142, 340 (2007).
R. Gnanasambandam and A. Protor, Food Chem., 68, 327 (2000).
F. T. Li, H. Yang, Y. Zhao and R. Xu, Chin. Chem. Lett., 18, 325 (2007).
G. Guibaud, N. Tixier, A. Bouju and M. Baudu, Chemosphere, 52, 1701 (2003).
R. Ashkenazy, L. Gottlieb and S. Yannai, Biotechnol. Bioeng., 55, 1 (1997).
P.X. Sheng, Y. P. Ting, J. P. Chen and L. Hong, J. Colloid Interface Sci., 275, 131 (2004).
S. Lagergren, K. Sven.vetenskapsakad. Handlingar, 24(4), 1 (1898).
Y. S. Ho, Ph.D. Thesis, Birmingham, UK, University of Birmingham (1995).
Y. S. Ho, Wat. Res., 40, 119 (2006).
C. Namasivayam and K. Ranganathan, Wat. Res., 29, 1737 (1995).
Z. Aksu, Sep. Purif. Technol., 21, 285 (2001).
N. Chubar, J. R. Carvalho and M. J. Neiva, Colloid and Surfaces B: Biointerfaces, 230, 57 (2004).
E.A. Deliyanni and K. A. Matis, Sep. Purif. Technol., 45, 96 (2005).
I. Langmuir, J. Am. Chem. Soc., 40, 1361 (1918).
H. M. F. Freundlich, J. Phys. Chem., 57, 385 (1906).
R. E. Treyball, Mass-transfer operations, 3rd Ed., McGraw Hill (1980).
O. Redlich and D. L. Peterson, J. Phys. Chem., 63, 1024 (1959).
C. Aharoni and M. Ungarish, J. Chem. Soc. Faraday Trans., 73, 456 (1977).
K.R. Hall, L.C. Eagleton, A. Acrivos and T. Vermeulen, Ind. Eng. Chem. Fundam., 5, 212 (1966).
K. K. Pandey, G. Prasad and V. N. Singh, Wat. Res., 19(7), 869 (1985).
K. S. Low, C. K. Lee and S. C. Liew, Process Biochem., 36, 59 (2000).
R. Leyva-Ramos, L.A. Bemal-Jacome and I. Acosta-Rodriguez, Sep. Puri. Technol., 45, 41 (2005).
M. Ajmal, R. A. K. Rao, S. Anwar, J. Ahmad and R. Ahmad, Bioresour. Technol., 86, 147 (2003).
I. Ghodbane, L. Nouri, O. Hamdaoui and M. Chiha, J. Hazard. Mater., 152(1), 148 (2007).
H. Benaissa and B. Benguella, Environ. Poll., 130, 157 (2004).
M. Mohapatra, T. Padhi, T. Dash, P. Singh, S. Anand and B. K. Mishra., Communicated to J. Hazard. Mater., (2009).
E. Pehlivan, B. H. Yanik, G. Ahmetli and M. Pehlivan, Bioresource Technol., 99, 3520 (2008).
K. S. Rao, S. Anand and P. Venkateswarlu, Bioresources, 5(1), 438 (2010).
D. Mohan, C.U. Pittman Jr. and P.H. Steele, J. Colloid Interface Sci., 297, 489 (2006).
A. Ozer and H. B. Pirincci, J. Hazard. Mater., B, 137, 849 (2006).
M. Martinez, N. Miralles, S. Hidalgo, N. Fiol, I. Villaescusa and J. Poch, J. Hazard. Mater., 133, 203 (2006).
Y. S. Ho and C. C. Wang, Process Biochem., 39, 759 (2004).
L. Nouri, I. Ghodbane, O. Hamdaoui and M. Chiha, J. Hazard. Mater., 149(1), 115 (2007).
S. Cay, A. Uyanýk and A.O. Zasýk, Sep. Purif. Technol., 38, 273 (2004).
M. Iqbal, A. Saeed and N. Akhtar, Bioresour. Technol., 81, 151 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rao, K.S., Anand, S. & Venkateswarlu, P. Cadmium removal from aqueous solutions using biosorbent Syzygium cumini leaf powder: Kinetic and equilibrium studies. Korean J. Chem. Eng. 27, 1547–1554 (2010). https://doi.org/10.1007/s11814-010-0243-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-010-0243-2