Abstract
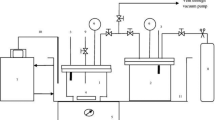
Modeling of solubility of acid gases in aqueous alkanolamine solutions is essential for design of an absorber for natural gas sweetening. In this work an apparatus similar to the device of Hayduk and Chen (1970), which was improved by Pahlavanzadeh and Motahhari (1997), for the measurement of gas solubility data by the synthetic method was used. The solubility of hydrogen sulfide in aqueous diisopropanolamine (DIPA) solution in mass concentration range of 30–40% for 101,325 Pa pressure and for temperature ranging from 313–343 K was reported. The obtained experimental solubility data of H2S in aqueous solutions of DIPA was used to predict the different interaction parameters of modified UNIQUAC-NRF model for calculating the activity coefficients. For nonideality of species in liquid phase, the UNIQUAC-NRF equation with ion-pair approach was applied. For long range interaction, the Pitzer-Debye-Huckel term was used.
Similar content being viewed by others
References
L. Kaewsichan and O. Al-Bofersen, Fluid Phase Equilibria, 183–184, 159 (2001).
A. Barreau, Oil & Gas Science and Technology — Rev. IFP, 61, 345 (2006).
F. Camacho, Ind. Eng. Chem. Res., 44, 7451 (2005).
Ezra E. Isaacs, Journal of Chemical and Engineering Data, 22, (1977).
W. Hayduk and S. c. Cheng, Can. J. Chem. Eng., 48, 93 (1970).
Iranian Journal of Chemistry and Chemical Engineering, 17, (1998).
Aspen plus, Aspen Technology, Inc., Ten Canal Park, Cambridge, MA 02141-22 01, USA, Ver. 10.2 (1997).
Perry, H. Robert, Perry’s Chemical Engineers’ Handbook (1984).
A. Henni, J. Chem. Eng. Data, 48, 1062 (2003).
C.-J. Hsieh, J.-M. Chen and M.-H. Li, J. Chem. Eng. Data, 52, 619 (2007).
A. Haghtalab and M. Dehghani Tafti, Application the UNIQUAC-NRF model to study the solubility of H 2 S & CO 2 in MEA & AMP, The 11th Iranian Chemical Engineering Congress (ICHEC11), November 28–30, Tehran, Iran (2006).
L. Kaewsichan, O. Al-Bofersen, V. F. Yesavage and M. S. Selim, Fluid Phase Equilibria, 183–184, 159 (2001).
A. Haghtalab and M. A. Asadolahi, Fluid Phase Equilibria, 171, 77 (2000).
K. S. Pitzer and J. M. Simonson, J. Phys. Chem., 90, 3005 (1986).
K. S. Pitzer, J. Phys. Chem., 77, 268 (1973).
W. Raatschen, A. H. Harvey and J. M. Prausnitz, Fluid Phase Equilibria, 38, 19 (1987).
W.-M. Qian, Y.-G. Li and A. E. Mather, Ind. Eng. Chem. Res., 34, 2545 (1995).
D. M. Austgen, G. T. Rochelle, X. Peng and C. C. Chen, Ind. Eng. Chem. Res., 28, 1060 (1989).
J. I. Lee, F.D. Otto and A. E. Mather, J. Chem. Eng. Data, 21, 207 (1976).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pahlavanzadeh, H., Farazar, M. Measurement and modeling of solubility of H2S in aqueous diisopropanolamine solution. Korean J. Chem. Eng. 26, 1112–1118 (2009). https://doi.org/10.1007/s11814-009-0185-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-009-0185-8