Abstract
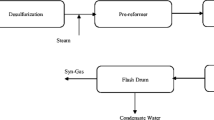
Carbon dioxide reforming of methane to syngas is one of the primary technologies of the new poly-generation energy system on the basis of gasification gas and coke oven gas. A free energy minimization is applied to study the influence of operating parameters (temperature, pressure and methane-to-carbon dioxide ratio) on methane conversion, products distribution, and energy coupling between methane oxidation and carbon dioxide reforming methane. The results show that the methane conversion increases with temperature and decreases with pressure. When the methane-to-carbon dioxide ratio increases, the methane conversion drops but the H2/CO ratio increases. By the introduction of oxygen, an energy balance in the process of the carbon dioxide reforming methane and oxidation can be realized, and the CO/H2 ratio can be adjusted as well without water-gas shift reaction for Fischer-Tropsch or methanol synthesis.
Similar content being viewed by others
References
Q. S. Jing, H. Lou, L. Y. Mo and X. M. Zheng, Energy Conversion and Management, 47, 459 (2006).
S. W. Nam, S. P. Yoon, H. Y. Ha, S. A. Hong and A. P. Maganyuk, Korean J. Chem. Eng., 17, 288 (2000).
Y. Matsumura and T. Nakamori, Applied Catalysis A: General, 1, 107 (2004).
J. Zhu, D. Zhang and K. D. King, Fuel, 80, 899 (2001).
K. H. Kim, S. Y. Lee and K. J. Yoon, Korean J. Chem. Eng., 23, 356 (2006).
B. B. Hwang, Y. K. Yeo and B. K. Na, Korean J. Chem. Eng., 20, 631 (2003).
R. Xiao, M. Zhang, B. Jin, Y. Huang and H. Zhou, Energy & Fuels, 20, 715 (2006).
W. Zhong and M. Zhang, AIChE J., 52, 924 (2006).
R. Xiao, M. Zhang, B. Jin and X. Liu, Can. J. Chem. Eng., 80, 800 (2002).
A. I. Tsyganok, M. Inaba, T. Tsunoda, S. Hamakawa, K. Suzuki and T. Hayakawa, Catalysis Communication, 4, 493 (2003).
V. A. Tsipouriari and X. E. Verkios, Catalysis Today, 64, 83 (2001).
S. J. Kong, H. J. Jin and Y. J. Ki, Korean J. Chem. Eng., 21, 793 (2004).
X. Li, J. R. Grace, A. P. Watkinson, C. J. Lim and A. Ergüdenler, Fuel, 80, 195 (2001).
W. Q. Zhang and Z. S. Zhang, Journal of Armory, 6, 812 (2005).
Y. Li, J. Xiao and M. Y. Zhang, Journal of Fuel Chemistry and Technology, 5, 556 (2005).
W. R. Smith and R. W. Missen, Chemical reaction equilibrium analysis: theory and algorithms, Wiley Publication, New York (1982).
Van F. Zegeren and S. H. Storey, The computation of chemical equilibria, Cambridge University Press, Cambridge (1970).
R. Holub and P. Vonka, The chemical equilibrium of gaseous systems, Reidel Publications, Dordrecht (1976).
Lisboa-Filho, Wido H. Schreiner, Edson Roberto Leite and Elson Longo, Applied Catalysis A: General, 2, 211 (2003).
Y. Matsumura and T. Nakamori, Applied Catalysis A: General, 1, 107 (2004).
V. R. Choudhary, A. M. Rajput and B. Prabhakar, Journal of Catalysis, 139, 326 (1993).
R. G. Ding, Z. F. Yan and L. Qian, Journal of Natural Gas Chemistry, 1, 50 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was presented at the 6th Korea-China Workshop on Clean Energy Technology held at Busan, Korea, July 4–7, 2006.
Rights and permissions
About this article
Cite this article
Li, Y., Jin, B. & Xiao, R. Carbon dioxide reforming of methane with a free energy minimization approach. Korean J. Chem. Eng. 24, 688–692 (2007). https://doi.org/10.1007/s11814-007-0027-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-007-0027-5