Abstract
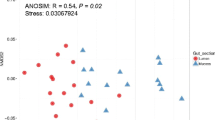
We used Illumina high-throughput sequencing of PCR-amplified V3-V4 16S rRNA gene regions to characterize bacterial communities associated with the adductor muscles, gills, gonads and intestines of the Yesso scallop (Patinopecten yessoensis) from waters around Zhangzidao, Dalian, China. Overall, 421,276 optimized reads were classified as 25 described bacterial phyla and 308 genera. Firmicutes, Proteobacteria, Tenericutes, Bacteroidetes, Chlamydiae and Spirochaetae accounted for > 97% of the total reads in the four organs. The bacterial 16S rDNA sequences assigned to Firmicutes and Proteobacteria were abundant in the adductor muscles, gills and gonads; while reads from Tenericutes were dominant in the intestines, followed by those from Firmicutes, Chlamydiae, Proteobacteria and Bacteroidetes. At the genus level, the dominant genera in the adductor muscles, gills and gonads appeared to be Bacillus, Enterococcus and Lactococcus, whereas Mycoplasma was dominant in the intestines. The relative abundances of Bacillus, Enterococcus, Lactococcus, Alkaliphilus, Raoultella, Paenibacillus and Oceanobacillus were significantly lower in the intestine than in the other three organs. Cluster analysis and principal coordinates analysis of the operational taxonomy units profile revealed significant differences in the bacterial community structure between the intestine and the other three organs. Taken together, these results suggest that scallops have intestine-specific bacterial communities and the adductor muscles, gills and gonads harbor similar communities. The difference in the bacterial community between organs may relate to unique habitats, surroundings, diet and their respective physiological functions.
Similar content being viewed by others
References
Abasolo–Pacheco, F., Saucedo, P. E., Mazón–Suástegui, J. M., Tovar–Ramírez, D., Araya, R., Ramírez–Orozco, J. M., and Campa–Córdova, Á. I., 2016. Isolation and use of beneficial microbiota from the digestive tract of lions–paw scallop Nodipecten subnodosus and winged pearl oyster Pteria sterna in oyster aquaculture. Aquaculture Research, 47: 3042–3051.
Ding, J., Dou, Y., Xu, G. R., Wang, Y. N., and Chang, Y. Q., 2014. Bacterial diversity in the mantle of Patinopecten yessoensis revealed by 454 pyrosequencing. Chinese Journal of Applied Ecology, 25: 3344–3328 (in Chinese with English abstract).
Dou, Y., Zhao, X. W., Ding, J., Liu, S. C., and Zhang, T., 2016. Application of high–throughput sequencing for analyzing bacterial diversity in the adductor muscle of sick and healthy Patinopecten yessoensis. Chinese Journal of Ecology, 35: 1019–1025 (in Chinese with English abstract).
Godoy, F. A., Espinoza, M., Wittwer, G., Uriarte, I., and Aranda, C., 2011. Characterization of culturable bacteria in larval cultures of the Chilean scallop Argopecten purpuratus. Ciencias Marinas, 37: 339–348.
Green, T. J., and Barnes, A. C., 2010. Bacterial diversity of the digestive gland of Sydney rock oysters, Saccostrea glomerata infected with the paramyxean parasite, Marteilia sydneyi. Journal of Applied Microbiology, 109: 613–622.
Hernández–Zárate, G., and Olmos–Soto, J., 2006. Identification of bacterial diversity in the oyster Crassostrea gigas by fluorescent in situ hybridization and polymerase chain reaction. Journal of Applied Microbiology, 100: 664–672.
Hine, P. M. and Diggles, B. K., 2002. Prokaryote infections in the new zealand scallops pecten novaezelandiae and chlamys delicatula. Diseases of Aquatic Organisms, 50: 137–144.
Holben, W. E., Williams. P., Saarinen, M., Särkilahti, L. K., and Apajalahti, J. H. A., 2002. Phylogenetic analysis of intestinal microflora indicates a novel Mycoplasma phylotype in farmed and wild salmon. Microbial Ecology, 44: 175–185.
Karim, M., Zhao, W.J., Rowley, D., Nelson, D., and Gomez–Chiarri, M., 2013. Probiotic strains for shellfish aquaculture: protection of eastern oyster, Crassostrea virginica, larvae and juveniles against bacterial challenge. Journal of Shellfish Research, 32: 401–408.
King, G. M., Judd, C., Kuske C. R., and Smith, C., 2012. Analysis of stomach and gut microbiomes of the Eastern oyster (Crassostrea virginica) from coastal louisiana, USA. PLoS One, 7: e51475.
Lasa, A., Mira, A., Camelo–Castillo, A., Belda–Ferre, P., and Romalde, J. L., 2016. Characterization of the microbiota associated to Pecten maximus gonads using 454–pyrosequencing. International Microbiology, 19: 91–397.
Li, M., Liu, J. C., Sun, X. Y., Zhao, X. W., Liang, J., Sun P. H., and Ma, Y. X., 2016. Culturable bacterial diversity in larval breeding in Yesso scallop Patinopecten yessoensis. Journal of Dalian Ocean University, 31: 374–3379 (in Chinese with English abstract).
Li, S., Sun, L., Wu, H., Hu, Z., Liu, W., Li, Y., and Wen, X., 2012. The intestinal microbial diversity in mud crab (Scylla paramamosain) as determined by PCR–DGGE and clone library analysis. Journal of Applied Microbiology, 113: 1341–1351.
Li, W. J. and Tan, K. F., 2009. The inspiration on massive mortality of scallop Patinopecten yessoensis resolved by Japan. Fisheries Science, 28: 609–612 (in Chinese with English abstract).
Liu, J. C., Sun, X. Y., Li M., Zhang, C. Y., Cao, S. Q., and Ma, Y. X., 2015. Vibrio infections associated with Yesso scallop (Patinopecten yessoensis) larval culture. Journal of Shellfish Research, 34: 213–216.
Liu, S. Y., Liu, J. R., Ma, Y. S., Shen, J., Yang, T. T., and Miao, Z. O., 2014. Intestinal flora in supply chain of live released Yesso scallop Patinopecten yessoensis. Fisheries Science, 33: 626–630 (in Chinese with English abstract).
Lu, G., Wang, F., Yu, Z., Lu, M., Wang, Y., Liu, C., Xue, Z., Wu, Y., Wang, L., and Song, L., 2017. Bacterial communities in gills and intestines of Yesso scallop (Patinopecten yessoensis) and its habitat waters in Changhai (Dalian, China). Invertebrate Survival Journal, 14: 340–351.
Meziti, A., Ramette, A., Mente, E., and Kormas, K. A., 2010. Temporal shifts of the Norway lobster (Nephrops norvegicus) gut bacterial communities. FEMS Microbiology Ecology, 74: 472–484.
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., Peplies, J., and Glöckner, F. O., 2013. The SILVA ribosomal RNA gene database project: Improved data processing and web–based tools. Nucleic Acids Research, 41: 590–596.
Riquelme, C., Chávez, P., Morales, Y. and Hayashida, G., 1994. Evidence for parental bacterial transfer to larvae in Argopecten purpuratus (Lamarck, 1819). Biological Research, 27: 129–134.
Riquelme, C., Hayashida, G., Vergara, N., Vasquez, A., Morales, Y., and Chavez, P., 1995. Bacteriology of the scallop Argopecten purpuratus, (lamarck, 1819) cultured in Chile. Aquaculture, 138: 49–60.
Romanenko, L. A., Uchino, M., Kalinovskaya, N. I., and Mikhailov, V. V., 2008. Isolation, phylogenetic analysis and screening of marine mollusc–associated bacteria for antimicrobial, hemolytic and surface activities. Microbiological Reserrch, 163: 633–644.
Romero, J., Garcia–Varela, M., Laclette, J. P., and Espejo, R. T., 2002. Bacterial 16S rRNA gene analysis revealed that bacteria related to Arcobacter spp. constitute an abundant and common component of the oyster microbiota (Tiostrea chilensis). Microbial Ecology, 44: 365–371.
Sandaa, R. A., Magnesen, T., Torkildsen, L., and Bergh, Ø., 2003. Characterisation of the bacterial community associated with early stages of great scallop, using denaturing gradient gel electrophoresis (DGGE). Systematic and Applied Microbiology, 26: 302–311.
Schulze, A. D., Alabi, A. O., Tattersall–Sheldrake, A. R., and Miller, K. M., 2006. Bacterial diversity in a marine hatchery: Balance between pathogenic and potentially probiotic bacterial strains. Aquaculture, 256: 50–73.
Sun, X. Y., Liu, J. C., Li, M., Zhao, X. W., Liang, J., Sun, P. H., Ma, Y. X. 2016. Characterization of bacterial communities associating with larval development of Yesso scallop (Patinopecten yessoensis Jay, 1857) by high–throughput sequencing. Journal of Ocean University of China, 15: 1067–1072.
Tanaka, R., Ootsubo, M., Sawabe, T., Ezura, Y., and Tajima, K. 2004. Biodiversity and in situ abundance of gut microflora of abalone (Haliotis discus hannai) determined by culture–independent techniques. Aquaculture, 241: 453–463.
Teng, W. M., LI, W. J., Zhang, M., Yu, Z. A., LI, S. L., Liu, X. F., Li H. L., and Fu, C. D., 2012. Isolation, identification and pathogenicity of Vibrio chagasii from Patinopecten yessoensis. Journal of Fisheries of China, 36: 937–943 (in Chinese with English abstract).
Trabal, N., Mazón–Suástegui, J. M., Vázquez–Juárez, R., Asencio–Valle, F., and Romero, J., 2014. Changes in the composition and diversity of the bacterial microbiota associated with oysters (Crassostrea corteziensis, Crassostrea gigas and Crassostrea sikamea) during commercial production. FEMS Microbiology Ecology, 88: 69–83.
Vaseeharan, B., and Ramasamy, P., 2003. Control of pathogenic Vibrio spp. by Bacillus subtilis BT23, a possible probiotic treatment for black tiger shrimp Penaeus monodon. Letters in Applied Microbiology, 36: 83–87.
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R., 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology, 73: 5261–5267.
Zhang, M. L., Sun, Y. H., Chen, L. Q., Cai, C. F., Qiao, F., Du, Z. Y., and Li, E. C., 2016. Symbiotic bacteria in gills and guts of Chinese mitten crab (Eriocheir sinensis) differ from the free–living bacteria in water. PLoS One, 11: e0148135.
Zurel, D., Benayahu, Y., Or, A., Kovacs A., and Gophna, U., 2011. Composition and dynamics of the gill microbiota of an invasive Indo–Pacific oyster in the eastern Mediterranean Sea. Environmental Microbiology, 13: 1467–1476.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, Y., Li, M., Sun, J. et al. Characterization of Bacterial Community Associated with Four Organs of the Yesso Scallop (Patinopecten yessoensis) by High-Throughput Sequencing. J. Ocean Univ. China 18, 493–500 (2019). https://doi.org/10.1007/s11802-019-3791-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-019-3791-z