Abstract
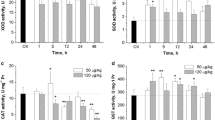
This study investigated the inductive effect of Alexandrium tamarense, a toxic dinoflagellate producing paralytic shellfish poison, on oxidative stress and apoptosis in hepatopancreas of Chinese shrimp, Fenneropenaeus chinensis. The individuals of F. chinensis were exposed to 200 and 1000 cells mL−1 of A. tamarense with their superoxide dismutase (SOD), glutathione S-transferase (GST) activities, malonyldialdehyde (MDA) concentration, and caspase gene (FcCasp) expression in hepatopancreas determined at 12, 24, 48, 72 and 96 h. In addition, apoptosis in hepatopancreas of F. chinensis at 96 h after exposure was determined through terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay. The hepatopancreatic SOD and GST activities of F. chinensis exposed to 1000 cells mL−1 of A. tamarense showed a bell-shaped response to exposure time. The hepatopancreatic MDA concentration of F. chinensis exposed to 1000 cells mL−1 of A. tamarense increased gradually from 48 to 96 h, and such a trend corresponded to the decrease of GST activity. The hepatopancreatic FcCasp transcript abundance of F. chinensis exposed to 1000 cells mL−1 of A. tamarense was positively and linearly correlated to MDA concentration. Results of TUNEL assay showed that exposure to 1000 cells mL−1 of A. tamarense induced apoptosis in the hepatopancreas of F. chinensis. Our study revealed that A. tamarense exposure influenced the antioxidative status of F. chinensis and caused lipid peroxidation and apoptosis in the hepatopancreas of shrimp.
Similar content being viewed by others
References
Alonso-Rodríguez, R., and Páez-Osuna, F., 2003. Nutrients, phytoplankton and harmful algal blooms in shrimp ponds: A review with special reference to the situation in the Gulf of California. Aquaculture, 219: 317–336.
Armstrong, J. S., 2006. Mitochondrial membrane permeabilization: The sine qua non for cell death. Bioessays, 28: 253–260.
Buikema, A. L., Niederlehner, B. R., and Cairns, J. J., 1982. Biological monitoring, part IV-toxicity testing. Water Research, 16: 239–262.
Buttke, T. M. and Sandstrom, P. A., 1994. Oxidative stress as a mediator of apoptosis. Immunology Today, 15: 7–10.
Chen, Y., 2008. Toxic effects and mechanisms of Diarrhetic Shellfish Poisoning (DSP) and other HAB toxins on mammalian cells. Ph.D thesis. Chinese Academy of Sciences, 60–69.
Costa, P. R., Botelho, M. J., and Lefebvre, K. A., 2010. Characterization of paralytic shellfish toxins in seawater and sardines (Sardina pilchardus) during blooms of Gymnodinium catenatum. Hydrobiologia, 655: 89–97.
Costa, P. R., Pereira, P., Guilherme, S., Barata, M., Nicolau, L., Santos, M. A., Pacheco, M., and Pousão-Ferreira, P., 2012. Biotransformation modulation and genotoxicity in white seabream upon exposure to paralytic shellfish toxins produced by Gymnodinium catenatum. Aquatic Toxicology, 106-107, 42–47.
Estrada, N., Romero, M. J., Campa-Córdova, A., Luna, A., and Ascencio, F., 2007. Effects of the toxic dinoflagellate, Gymnodinium catenatum on hydrolytic and antioxidant enzymes, in tissues of the giant lions-paw scallop Nodipecten subnodosus. Comparative Biochemistry and Physiology, 146: 502–510.
Fleury, C., Mignotte, B., and Vayssiere, J. L., 2002. Mitochondrial reactive oxygen species in cell death signaling. Biochimie, 84: 131–141.
García, C., Bravo, M. C., Lagos, M., and Lagos, N., 2004. Paralytic shellfish poisoning: Post mortem analysis of tissue and body fluid samples from human victims in the patagonia fjords. Toxicon, 43: 149–158.
Gubbins, M. J., Eddy, F. B., Gallacher, S., and Stagg, R. M., 2000. Paralytic shellfish poisoning toxins induce xenobiotic metabolishing enzymes in Atlantic salmon (Salmo salar). Marine Environmental Research, 50: 479–483.
Jeon, J. K., Lee, J. S., Shim, W. J., Aarakawa, O., Takatani, T., Honda, S., and Noguchi, T., 2008. Changes in activity of hepatic xenobiotic-metabolizing enzymes of tiger puffer (Takifugu rubripes) exposed to paralytic shellfish poisoning toxins. Journal of Environmental Biology, 29: 599–603.
Leu, J. H., Wang, H. C., Kou, G. H., and Lo, C. F., 2008. Penaeus monodon caspase is targeted by a white spot syndrome virus anti-apoptosis protein. Developmental and Comparative Immunology, 32: 476–486.
Li, Y. Q., Li, J., and Wang, Q. Y., 2006. The effects of dissolved oxygen concentration and stocking density on growth and non-specific immunity factors in Chinese shrimp, Fennero-penaeus chinensis. Aquaculture, 256: 608–616.
Lin, Y. S., 1996. Red tide caused by a marine toxic dinoflagellate, Alexandrium tamarensis (Lebour) Baleon, in shrimp ponds in Xiamen. Taiwan Strait, 15: 16–18 (in Chinese).
Livingstone, D. R., 2001. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Marine Pollution Bulletin, 42: 656–666.
Livingstone, D. R., 2003. Oxidative stress in aquatic organisms in relation to pollution and aquaculture. Revue De Medecine Veterinaire, 154: 427–430.
Marisa, L. W., and Juan, F. M., 2005. Real-time PCR for mRNA quantitation. Biotechniques, 39: 75–85.
Ron, V. D. O., Jonny, B., and Nico, P. E. V., 2003. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environmental Toxicology and Pharmacology, 13: 57–149.
Sakamoto, S., Sato, S., Ogata, T., and Kodama, M., 2000. Formation of intermediate conjugates in the reductive transformation of gonyautoxins to saxitoxins by thiolcompounds. Fisheries Science, 66: 136–141.
Sato, S., Sakai, R., and Kodama, M., 2000. Identification of thioether intermediates in the reductive transformation of gonyautoxins into saxitoxins by thiols. Bioorganic & Medicinal Chemistry Letters, 10: 1787–1789.
Skulachev, V. P., 1998. Cytochromec in the apoptotic and antioxidant cascades. Febs Letters, 423: 275–280.
Stephan, P., Claudia, W., Stephanie, W., Helge, S., and Harri, K., 2005. Activity and substrate specificity of cytosolic and microsomal glutathione S-transferase in Australian black tiger prawns (Penaeus monodon) after exposure to cyanobacterial toxins. Environmental Toxicology, 20: 301–307.
Su, H. M., Liao, I. C., and Chiang, Y. M., 1993. Mass mortality of prawn caused by Alexandrium tamarense blooming in a culture pond in southern Taiwan. In: Toxic Phytoplankton Blooms in the Sea. Smayda, T. J., and Shimizu, Y., eds., Elsevier Science Publishers B V, Amsterdam, 329–333.
Tan, Z. J., Yan, T., Zhou, M. J., Li, J., Yu, R. C., and Wang, Y. F., 2002. The effects of Alexandrium tamarense on survival, growth and reproduction of Neomysis awatschensis. Acta Ecologica Sinica, 22: 1635–1639.
Sun, Y., Yin, G., Zhang, J., Yu, H., and Wang, X., 2007. Bioaccumulation and ROS generation in liver of freshwater fish, goldfish Carassius auratus under HC Orange No.1 exposure. Environmental Toxicology, 22: 256–263.
Tan, Z. J., 2006. Toxic effects and its mechanism of dinoflagellate Alexandrium tamarense on perch Lateolabrax japonicus. Ph.D thesis. Chinese Academy of Sciences, 24–34.
Tan, Z. J., Yan, T., and Yu, R. C., 2007. Transfer of paralytic shellfish toxins via marine food chains: A simulated experiment. Biomedical and Environmental Sciences, 20: 235–241.
Thornberry, N. A., 1998. Caspases: Key mediators of apoptosis. Chemistry and Biology, 5: 97–103.
Trezado, C., Hidalgo, M. C., García-Gallego, M., Morales, A. E., Furne, M., Domezain, A., Domezain, J., and Sanz, A., 2006. Antioxidant enzymes and lipid peroxidation in sturgeon Acipenser naccarii and trout Oncorhynchus mykiss. A comparative study. Aquaculture, 254: 758–767.
Wang, W., and Ballatori, N., 1998. Endogenous glutathione conjugates: Occurrence and biological functions. Pharmacological Reviews, 50: 335–352.
Wang, Y., Li, J., Liu, P., Li, J. T., Zhang, Z., Chang, Z. Q., He, Y. Y., and Liu, D. Y., 2011. The responsive expression of a caspase gene in Chinese shrimp Fenneropenaeus chinensis against pH stress. Aquaculture Research, 42: 1214–1230.
Winston, G. W., 1991. Oxidants and antioxidants in aquatic animals. Comparative Biochemistry and Physiology Part C Comparative Pharmacology, 100: 173–176.
Xu, W. N., Liu, W. B., and Liu, Z. P., 2009. Trichlorfon-induced apoptosis in hepatocyte primary cultures of Carassius auratus gibelio. Chemosphere, 77: 895–901.
Yan, T., Zhou, M. J., Fu, M., Yu, R. C., Wang, Y. F., and Li, J., 2003. Effects of the dinoflagellate Alexandrium tamarense on early development of the Scallop Argopectan irradians concentricus. Aquaculture, 217: 167–178.
Zhu, M. Y., 1993. Red tide in shrimp ponds along the Bohai Sea. In: Toxic Phytoplankton Blooms in the Sea. Smayda, T. J., and Shimizu, Y., eds., Elsevier Science Publishers B V, Amsterdam, 363–367.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, Z., Li, J., Li, J. et al. Toxic dinoflagellate Alexandrium tamarense induces oxidative stress and apoptosis in hepatopancreas of shrimp (Fenneropenaeus chinensis). J. Ocean Univ. China 13, 1005–1011 (2014). https://doi.org/10.1007/s11802-014-2397-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11802-014-2397-8