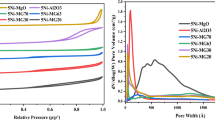
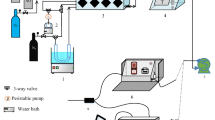
Abstract
To decompose efficiently hydrogen cyanide (HCN) in exhaust gas, g-Al2O3-supported bimetallicbased Cu–Ni catalyst was prepared by incipient-wetness impregnation method. The effects of the calcination temperature, H2O/HCN volume ratio, reaction temperature, and the presence of CO or O2 on the HCN removal efficiency on the Cu–Ni/g-Al2O3 catalyst were investigated. To examine further the efficiency of HCN hydrolysis, degradation products were analyzed. The results indicate that the HCN removal efficiency increases and then decreases with increasing calcination temperature and H2O/HCN volume ratio. On catalyst calcined at 400°C, the efficiency reaches a maximum close to 99% at 480 min at a H2O/HCN volume ratio of 150. The HCN removal efficiency increases with increasing reaction temperature within the range of 100°C–500°C and reaches a maximum at 500°C. This trend may be attributed to the endothermicity of HCN hydrolysis; increasing the temperature favors HCN hydrolysis. However, the removal efficiencies increases very few at 500°C compared with that at 400°C. To conserve energy in industrial operations, 400°C is deemed as the optimal reaction temperature. The presence of CO facilitates HCN hydrolysis andincreases NH3 production. O2 substantially increases the HCN removal efficiency and NO x production but decreases NH3 production.

Similar content being viewed by others

References
Duquesne S, Bars M L, Bourbigot S, Delobel R, Poutch F, Camino G, Eling B, Lindsay C, Roels T. Analysis of fire gases released from polyurethane and fire-retarded polyurethane coatings. Journal of Fire Sciences, 2000, 18(6): 456–482
Dagaut P, Glarborg P, Alzueta M U. The oxidation of hydrogen cyanide and related chemistry. Progress in Energy and Combustion Science, 2008, 34(1): 1–46
Tuovinen H, Blomqvist P, Saric F. Modelling of hydrogen cyanide formation in room fires. Fire Safety Journal, 2004, 39(8): 737–755
Karlsson H L. Ammonia, nitrous oxide and hydrogen cyanide emissions from five passenger vehicles. Science of the Total Environment, 2004, 334-335: 125–132
Yuan S, Zhou Z J, Li J, Chen X L, Wang F C. HCN and NH3 released from biomass and soybean cake under rapid pyrolysis. Energy & Fuels, 2010, 24(11): 6166–6171
Wang Z H, Jiang M, Ning P, Xie G. Thermodynamic modeling and gaseous pollution prediction of the yellow phosphorus production. Industrial & Engineering Chemistry Research, 2011, 50(21): 12194–12202
Jiang M, Wang Z H, Ning P, Tian S L, Huang X F, Bai Y W, Shi Y, Ren X G, Chen W, Qin Y S, Zhou J, Miao R R. Dust removal and purification of calcium carbide furnace off-gas. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(3): 901–907
Zhang F M, Li K X, Lu C X, Lu Y G, Yang Y G, Ling L C. Removal methods of hydrogen cyanide. New Carbon Materials, 2003, 18(2): 151–157
Jiang M, Ning P, Wang C H, Chen W, Zhou J, Wang L, Qin Y S. Research progress of HCN-containing exhaust gas treatment. Chemical Industry and Engineering Progress, 2012, 31(11): 2563–2569
Ning P, Jiang M, Wang X Q, Yang H, Shi Y, Bo Y W. Adsorption of low-concentration HCN on impregnated activated carbon. Journal of Chemical Engineering of Chinese Universities, 2010, 24(6): 1038–1045
Ye P W, Luan Z Q, Li K, Yu L Q, Zhang J C. The use of a combination of activated carbon and nickel microfibers in the removal of hydrogen cyanide from air. Carbon, 2009, 47(7): 1799–1805
Ning P, Qiu J, Wang X, Liu W, Chen W. Metal loaded zeolite adsorbents for hydrogen cyanide removal. Journal of Environmental Sciences (China), 2013, 25(4): 808–814
Colín G M, Ortega G F, Ramos B S, Negron M A. Heterogeneous radiolysis of HCN adsorbed on a solid surface. Nuclear Instruments & Methods in Physics Research. Section A, Accelerators, Spectrometers, Detectors and Associated Equipment, 2010, 619(1): 83–85
Tan H Z, Wang X B, Wang C L, Xu T M. Characteristics of HCN removal using CaO at high temperatures. Energy & Fuels, 2009, 23(3): 1545–1550
Zhao H B, Tonkyn R G, Barlow S E, Koel B E, Peden C H F. Catalytic oxidation of HCN over a 0.5% Pt/Al2O3 catalyst. Applied Catalysis B: Environmental, 2006, 65(3): 282–290
Giménez-López J, Miller A, Bilbao R, Alzueta MU. HCN oxidation in an O2/CO2 atmosphere: an experimental and kinetic modeling study. Combustion and Flame, 2010, 157(2): 267–276
Marsh J D F, Newling W B S, Rich J. The catalytic hydrolysis of hydrogen cyanide to ammonia. Journal of Applied Chemistry, 1952, 2(12): 681–684
Nanba T, Obuchi A, Akaratiwa S, Liu S, Uchisawa J, Kushiyama S. Catalytic hydrolysis of HCN over H-ferrierite. Chemistry Letters, 2000, 29(9): 986–987
Schäfer S, Bonn B. Hydrolysis of HCN as an important step in nitrogen oxide formation in fluidised combustion. Part II: Heterogeneous reactions involving limestone. Fuel, 2012, 81(13): 1641–1646
Kröcher O, Elsener M. Hydrolysis and oxidation of gaseous HCN over heterogeneous catalysts. Applied Catalysis B: Environmental, 2009, 92(1–2): 75–89
Yuan X, Zhou J, Tian S L. Effects of transition metals grafted gamma-Al2O3 on catalytic hydrolysis of HCN. Research of Environmental Sciences, 2014, 27(12): 1465–1471
Gayán P, Dueso C, Abad A, Adanez J, Diego L F. NiO/Al2O3 oxygen carriers for chemical-looping combustion prepared by impregnation and deposition-precipitation methods. Fuel, 2009, 88(6): 1016–1023
Gayán P, Diego L F, García-Labiano F. Effect of support on reactivity and selectivity of Ni-based oxygen carriers for chemicallooping combustion. Fuel, 2008, 87(12): 2641–2650
HJ 484–2009, Water quality-determination of cyanid-volumetric and spectro-photometry method
Rida K, Benabbas A, Bouremmad F, Peña M A, Sastre E, Martínez- Arias A. Effect of calcination temperature on the structural characteristics and catalytic activity for propene combustion of sol-gel derived lanthanum chromite perovskite. Applied Catalysis A, General, 2007, 327(2): 173–179
Cheng W, Xu J, Ding W, Wang Y, Zheng W, Wu F, Li J. Synthesis of porous super paramagnetic iron oxides from colloidal nanoparticles: effect of calcination temperature and atmosphere. Materials Chemistry and Physics, 2015, 153: 187–194
Das T, Sengupta S, Deo G. Effect of calcination temperature during the synthesis of Co/Al2O3 catalyst used for the hydrogenation of CO2. Reaction Kinetics, Mechanisms and Catalysis, 2013, 110(1): 147–162
Zhang N W, Huang C J, Zhu X Q, Xu J D, Weng W Z, Wan H L. Effect of calcination temperature and pretreatment with reaction gas on properties of Co/g-Al2O3 catalysts for partial oxidation of methane. Chemistry, an Asian Journal, 2012, 7(8): 1895–1901
Jung J S, Lee J S, Choi G, Ramesh S, Moon D J. The characterization of micro-structure of cobalt on g-Al2O3 for FTS: effects of pretreatment on Ru-Co/g-Al2O3. Fuel, 2015, 149: 118–129
Wang J H, Dong X D, Wang Y J, Li Y. Effect of the calcination temperature on the performance of a CeMoOx. Catalysis Today, 2015, 245: 10–15
Zhu J, Wang W, Hua X N, Xia Z, Deng Z. Simultaneous CO2 capture and H2 generation using Fe2O3/Al2O3 and Fe2O3/CuO/ Al2O3 as oxygen carriers in single packed bed reactor via chemical looping process. Frontiers of Environmental Science & Engineering, 2015, 9(6): 1117–1129
Ko E Y, Park E D, Seo K W, Lee H C, Lee D, Kim S. Pt-Ni/g-Al2O3 catalyst for the preferential CO oxidation in the hydrogen stream. Catalysis Letters, 2006, 110(3–4): 275–279
Yang X C, Lu Z G, Kang X C, Wei Y N. Effect of ZrO2 on the structure of NiO/CeO2/g-Al2O3 composite catalysts. Journal of Inorganic Materials, 2009, 24(1): 187–191
Kröcher O, Elsener M, Jacob E. A model gas study of ammonium formate, methanamide and guanidinium formate as alternative ammonia precursor compounds for the selective catalytic reduction of nitrogen oxides in diesel exhaust gas. Applied Catalysis B: Environmental, 2009, 88(1–2): 66–82
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yan, L., Tian, S., Zhou, J. et al. Catalytic hydrolysis of gaseous HCN over Cu–Ni/γ-Al2O3 catalyst: parameters and conditions. Front. Environ. Sci. Eng. 10, 5 (2016). https://doi.org/10.1007/s11783-016-0872-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11783-016-0872-8