Abstract
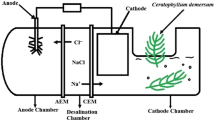
Microbial desalination cell (MDC) is a promising technology to desalinate water and generate electrical power simultaneously. The objectives of this study were to investigate the desalination performance of monovalent and divalent cations in the MDC, and discuss the effect of ion characteristics, ion concentrations, and electrical characteristics. Mixed salt solutions of NaCl, MgCl2, KCl, and CaCl2 with the same concentration were used in the desalination chamber to study removal of cations. Results showed that in the mixed salt solutions, the electrodialysis desalination rates of cations were: Ca2+ >Mg2+>Na+>K+. Higher ionic charges and smaller hydrated ionic radii resulted in higher desalination rates of the cations, in which the ionic charge was more important than the hydrated ionic radius. Mixed solutions of NaCl and MgCl2 with different concentrations were used in the desalination chamber to study the effect of ion concentrations. Results showed that when ion concentrations of Na+ were one-fifth to five times of Mg2+, ion concentration influenced the dialysis more profoundly than electrodialysis. With the current densities below a certain value, charge transfer efficiencies became very low and the dialysis was the main process responsible for the desalination. And the phosphate transfer from the anode chamber and potassium transfer from the cathode chamber could balance 1%–3% of the charge transfer in the MDC.
Similar content being viewed by others
References
Cao X, Huang X, Liang P, Xiao K, Zhou Y, Zhang X, Logan B E. A new method for water desalination using microbial desalination cells. Environmental Science & Technology, 2009, 43(18): 7148–7152
Logan B E, Regan J M. Microbial fuel cells-challenges and applications. Environmental Science & Technology, 2006, 40(17): 5172–5180
Hou Y, Li K, Luo H, Liu G, Zhang R, Qin B, Chen S. Using crosslinked polyvinyl alcohol polymer membrane as a separator in the microbial fuel cell. Frontiers of Environmental Science and Engineering
Chen X, Xia X, Liang P, Cao X, Sun H, Huang X. Stacked microbial desalination cells to enhance water desalination efficiency. Environmental Science & Technology, 2011, 45(6): 2465–2470
Jacobson K S, Drew D M, He Z. Efficient salt removal in a continuously operated upflow microbial desalination cell with an air cathode. Bioresource Technology, 2011, 102(1): 376–380
Kim Y, Logan B E. Series assembly of microbial desalination cells containing stacked electrodialysis cells for partial or complete seawater desalination. Environmental Science & Technology, 2011, 45(13): 5840–5845
Luo H, Jenkins P E, Ren Z. Concurrent desalination and hydrogen generation using microbial electrolysis and desalination cells. Environmental Science & Technology, 2011, 45(1): 340–344
Mehanna M, Kiely P D, Call D F, Logan B E. Microbial electrodialysis cell for simultaneous water desalination and hydrogen gas production. Environmental Science & Technology, 2010, 44(24): 9578–9583
Chen S, Liu G, Zhang R, Qin B, Luo Y. Development of the microbial electrolysis desalination and chemical-production cell for desalination as well as acid and alkali productions. Environmental Science & Technology, 2012, 46(4): 2467–2472
Chen S, Liu G, Zhang R, Qin B, Luo Y, Hou Y. Improved performance of the microbial electrolysis desalination and chemicalproduction cell using the stack structure. Bioresource Technology, 2012, 116: 507–511
Jacobson K S, Drew D M, He Z. Use of a liter-scale microbial desalination cell as a platform to study bioelectrochemical desalination with salt solution or artificial seawater. Environmental Science & Technology, 2011, 45(10): 4652–4657
Mehanna M, Saito T, Yan J L, Hickner M, Cao X, Huang X, Logan B E. Using microbial desalination cells to reduce water salinity prior to reverse osmosis. Energy & Environmental Science, 2010, 3(8): 1114–1120
Cheng S, Xing D, Call D F, Logan B E. Direct biological conversion of electrical current into methane by electromethanogenesis. Environmental Science & Technology, 2009, 43(10): 3953–3958
Liu H, Cheng S, Logan B E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environmental Science & Technology, 2005, 39(2): 658–662
Logan B E, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K. Microbial fuel cells: methodology and technology. Environmental Science & Technology, 2006, 40(17): 5181–5192
Firdaous L, Quéméneur F, Schlumpf J P, Malériat J P. Modification of the ionic composition of salt solutions by electrodialysis. Desalination, 2004, 167: 397–402
Walha K, Amar R B, Firdaous L, Quéméneur F, Jaouen P. Brackish groundwater treatment by nanofiltration, reverse osmosis and electrodialysis in Tunisia: performance and cost comparison. Desalination, 2007, 207(1–3): 95–106
Luo H, Xu P, Jenkins P E, Ren Z. Ionic composition and transport mechanisms in microbial desalination cells. Journal of Membrane Science, 2012, 409–410: 16–23
Kim J R, Cheng S, Oh S E, Logan B E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environmental Science & Technology, 2007, 41(3): 1004–1009
Choi J H, Lee H J, Moon S H. Effects of electrolytes on the transport phenomena in a cation-exchange membrane. Journal of Colloid and Interface Science, 2001, 238(1): 188–195
Rozendal R A, Sleutels T H J A, Hamelers H V M, Buisman C J N. Effect of the type of ion exchange membrane on performance, ion transport, and pH in biocatalyzed electrolysis of wastewater. Water Science and Technology, 2008, 57(11): 1757–1762
Elattar A, Elmindaoui A, Pismenskaia N, Gavach C, Pourcelly G. Comparison of transport properties of monovalent anions through anion-exchange membranes. Journal of Membrane Science, 1998, 143(1–2): 249–261
Sleutelsa T H J A, Hamelersa H V M, Rozendal R A, Buisman C J N. Ion transport resistance in microbial electrolysis cells with anion and cation exchange membranes. International Journal of Hydrogen Energy, 2009, 34(9): 3612–3620
Rozendal R A, Hamelers H V M, Molenkamp R J, Buisman C J N. Performance of single chamber biocatalyzed electrolysis with different types of ion exchange membranes. Water Research, 2007, 41(9): 1984–1994
Fan Y, Hu H, Liu H, Sustainable power generation in microbial fuel cells using bicarbonate buffer and proton transfer mechanisms. Environmental Science & Technology, 2007, 41(23): 8154–8158
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Luo, H., Hou, Y. et al. Comparison of the removal of monovalent and divalent cations in the microbial desalination cell. Front. Environ. Sci. Eng. 9, 317–323 (2015). https://doi.org/10.1007/s11783-013-0596-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-013-0596-y