Abstract
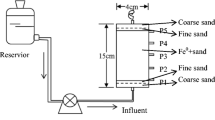
The reduction of hexavalent chromium by scrap iron was investigated in continuous long-term fixed bed system. The effects of pH, empty bed contact time (EBCT), and initial Cr(VI) concentration on Cr(VI) reduction were studied. The results showed that the pH, EBCT, and initial Cr(VI) concentration significantly affected the reduction capacity of scrap iron. The reduction capacity of scrap iron were 4.56, 1.51, and 0.57 mg Cr(VI)·g−1 Fe0 at pH 3, 5, and 7 (initial Cr(VI) concentration 4 mg·L−1, EBCT 2 min, and temperature 25°C), 0.51, 1.51, and 2.85 mg Cr(VI)·g−1 Fe0 at EBCTs of 0.5, 2.0, and 6.0 min (initial Cr(VI) concentration 4mg·L−1, pH 5, and temperature 25°C), and 2.99, 1.51, and 1.01 mg Cr(VI)·g−1 Fe0 at influent concentrations of 1, 4, and 8 mg·L−1 (EBCT 2 min, pH 5, and temperature 25°C), respectively. Fe(total) concentration in the column effluent continuously decreased in time, due to a decrease in time of the iron corrosion rate. The fixed bed reactor can be readily used for the treatment of drinking water containing low amounts of Cr(VI) ions, although the hardness and humic acid in water may shorten the lifetime of the reactor, the reduction capacity of scrap iron still achieved 1.98 mg Cr6 +·g−1 Fe. Scanning electron microscope equipped with energy dispersion spectrometer and X-ray diffraction were conducted to examine the surface species of the scrap iron before and after its use. In addition to iron oxides and hydroxide species, iron-chromium complex was also observed on the reacted scrap iron.
Similar content being viewed by others
References
Schlautman M A, Han I. Effects of pH and dissolved oxygen on the reduction of hexavalent chromium by dissolved ferrous iron in poorly buffered aqueous systems. Water Research, 2001, 35(6): 1534–1546
Wielinga B, Mizuba M M, Hansel C M, Fendorf S. Iron promoted reduction of chromate by dissimilatory iron-reducing bacteria. Environmental Science & Technology, 2001, 35(3): 522–527
Costa M. Potential hazards of hexavalent chromate in our drinking water. Toxicology and Applied Pharmacology, 2003, 188(1): 1–5
Wang X J, Wang Y, Wang X, Liu M, Xia S Q, Yin D Q, Zhang Y L, Zhao J F. Microwave-assisted preparation of bamboo charcoal-based iron-containing adsorbents for Cr(VI) removal. Chemical Engineering Journal, 2011, 174(1): 326–332
Zhou H, He Y, Lan Y, Mao J, Chen S. Influence of complex reagents on removal of chromium(VI) by zero-valent iron. Chemosphere, 2008, 72(6): 870–874
Pellerin C, Booker S M. Reflections on hexavalent chromium: health hazards of an industrial heavyweight. Environmental Health Perspectives, 2000, 108(9): a402–a407
Blowes D W, Ptacek C J, Jambor J L. In-situ remediation of Cr (VI)-contaminated groundwater using permeable reactive walls: laboratory studies. Environmental Science & Technology, 1997, 31(12): 3348–3357
Puls R W, Blowes D W, Gillham R W. Long-term performance monitoring for a permeable reactive barrier at the U.S. Coast Guard Support Center, Elizabeth City, North Carolina. Journal of Hazardous Materials, 1999, 68(1–2): 109–124
Puls R W, Paul C J, Powell R M. The application of in situ permeable reactive (zero-valent iron) barrier technology for the remediation of chromate-contaminated groundwater: a field test. Applied Geochemistry, 1999, 14(8): 989–1000
Blowes D W, Ptacek C J, Benner S G, McRae C W T, Bennett T A, Puls R W, Bennett T A, Puls R W. Treatment of inorganic contaminants using permeable reactive barriers. Journal of Contaminant Hydrology, 2000, 45(1–2): 123–137
Terry P A. Characterization of Cr ion exchange with hydrotalcite. Chemosphere, 2004, 57(7): 541–546
Siegel S K, Clifford D A. Removal of Chromium from Ion Exchange Regenerant Solution, US EPA, Water Engineering Laboratory, EPA/600/S2-88/ 007. Houston: US Environmental Protection Agency, 1988
Sengupta A K, Clifford D. Important process variables in chromate ion exchange. Environmental Science & Technology, 1986, 20(2): 149–155
Ölmez T. The optimization of Cr(VI) reduction and removal by electrocoagulation using response surface methodology. Journal of Hazardous Materials, 2009, 162(2–3): 1371–1378
Gode F, Pehlivan E. Removal of Cr(VI) from aqueous solution by two Lewatit-anion exchange resins. Journal of Hazardous Materials, 2005, 119(1–3): 175–182
Bhowal A, Datta S. Studies on transport mechanism of Cr(VI) extraction from an acidic solution using liquid surfactant membranes. Journal of Membrane Science, 2001, 188(1): 1–8
Çengeloğlu Y, Tor A, Kir E, Ersoz M. Transport of hexavalent chromium through anion-exchange membranes. Desalination, 2003, 154(3): 239–246
Singh D K, Bharadwaj R K, Srivastava B, Sahu A. Extraction of hexavalent chromium from aqueous solution by emulsion liquid membrane. Journal of Scientific and Industrial Research, 2002, 61(7): 538–542
Francisco J A, Concepción C, María I M. Transport of chromium (VI) through a Cyanex 921-supported liquid membrane from HCl solutions. Journal of Chemical Technology and Biotechnology (Oxford, Oxfordshire), 2003, 78(10): 1048–1053
Yu L J, Shukla S S, Dorris K L, Shukla A, Margrave J L. Adsorption of chromium from aqueous solutions by maple sawdust. Journal of Hazardous Materials, 2003, 100(1–3): 53–63
Daneshvar N, Salari D, Aber S. Chromium adsorption and Cr(VI) reduction to trivalent chromium in aqueous solutions by soya cake. Journal of Hazardous Materials, 2002, 94(1): 49–61
Han I, Schlautman M A, Batchelor B. Removal of hexavalent chromium from groundwater by granular activated carbon. Water Environment Research, 2000, 72(1): 29–39
Perezcandela M, Martinez J M M, Maciz R T. Chromium(VI) removal with activated carbons. Water Research, 1995, 29(9): 2174–2180
Eary L E, Rai D. Chromate removal from aqueous wastes by reduction with ferrous ion. Environmental Science & Technology, 1988, 22(8): 972–977
Erdem M, Tumen F. Chromium removal from aqueous solution by the ferrite process. Journal of Hazardous Materials, 2004, 109(1–3): 71–77
Higgins T E, Sater V E. Combined removal of Cr, Cd and Ni from wastes. Environment and Progress, 1984, 3(1): 12–25
Öh B T, Just C L, Alvarez P J J. Hexahydro-1,3,5-trinitro-1,3,5-triazine mineralization by zerovalent iron and mixed anaerobic cultures. Environmental Science & Technology, 2001, 35(21): 4341–4346
Hung H M, Ling F H, Hoffmann M R. Kinetics and mechanism of the enhanced reductive degradation of nitrobenzene by elemental iron in the presence of ultrasound. Environmental Science & Technology, 2000, 34(9): 1758–1763
Kim Y H, Carraway E R. Dechlorination of pentachlorophenol by zero-valent iron and modified zero-valent iron. Environmental Science & Technology, 2000, 34(10): 2014–2017
Alowitz M J, Scherer M M. Kinetics of nitrate, nitrite, and Cr(VI) reduction by iron metal. Environmental Science & Technology, 2002, 36(3): 299–306
Chang L Y. Chromate reduction in wastewater at different Ph levels using thin iron wires-a laboratory study. Environment and Progress, 2005, 24(3): 305–316
Lo I M C, Lam C S C, Lai K C K. Hardness and carbonate effects on the reactivity of zero-valent iron for Cr(VI) removal. Water Research, 2006, 40(3): 595–605
Lo I M C, Lam C S C, Lai K C K. Competitive effects of trichloroethylene on Cr(VI) removal by zero-valent iron. Journal of Environmental Engineering, 2005, 131(11): 1598–1606
Gheju M, Iovi A. Kinetics of hexavalent chromium reduction by scrap iron. Journal of Hazardous Materials, 2006, 135(1–3): 66–73
Gheju M, Iovi A, Balcu I. Hexavalent chromium reduction with scrap iron in continuous-flow system. Journal of Hazardous Materials, 2008, 153(1–2): 655–662
Özer A, Altundoğan H S, Erdem M, Tümen F. A study on the Cr(VI) removal from aqueous solutions by steel wool. Environmental pollution, 1997, 97(1–2): 107–112
American Public Health Association (APHA), American Water Works Association (AWWA), Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater. 21st ed. Baltimore, MD: Americn Public Health Association, 2005
Chen S S, Hsu B C, Hung L W. Chromate reduction by waste iron from electroplating wastewater using plug flow reactor. Journal of Hazardous Materials, 2008, 152(3): 1092–1097
Ministry of Health of the People’s Republic of China, Standardization Administration of the People’s Republic of China. Chinese Standards for Drinking Water Quality (GB5749-2006). Beijing: Ministry of Health of the People’s Republic of China & Standardization Administration of the People’s Republic of China, 2006
Chen S S, Cheng C Y, Li C W, Chai P H, Chang Y M. Reduction of chromate from electroplating wastewater from pH 1 to 2 using fluidized zero valent iron process. Journal of Hazardous Materials, 2007, 142(1–2): 362–367
Melitas N, Chuffe-Moscoso O, Farrell J. Kinetics of soluble chromium removal from contaminated water by zerovalent iron media: corrosion inhibition and passive oxide effects. Environmental Science & Technology, 2001, 35(19): 3948–3953
Gheju M, Balcu I. Hexavalent chromium reduction with scrap iron in continuous-flow system. Part 2: effect of scrap iron shape and size. Journal of Hazardous Materials, 2010, 182(1–3): 484–493
Liu T Z, Tsang D C W, Lo I M C. Chromium(VI) reduction kinetics by zero-valent iron in moderately hard water with humic acid: iron dissolution and humic acid adsorption. Environmental Science & Technology, 2008, 42(6): 2092–2098
Erdem M, Altundogan H S, Özer A, Tümen F. Cr(VI) reduction in aqueous solutions by using synthetic iron sulphide. Environmental Technology, 2001, 22(10): 1213–1222
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, X., Wang, X. et al. Reduction of hexavalent chromium with scrap iron in a fixed bed reactor. Front. Environ. Sci. Eng. 6, 761–769 (2012). https://doi.org/10.1007/s11783-012-0413-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11783-012-0413-z