Abstract
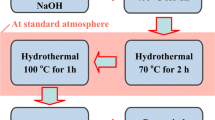
Chitosan-coated fly ash (CWF) was prepared by the acid leaching-coating method. Chitosan and fly ash were crosslinked in the solution of acetic acid and sulfuric acid. The microstructure of CWF was conducted by scanning electron microscope (SEM) and X-ray diffraction (XRD). The removal of Cr(VI) from water by CWF was studied by adsorption experiments. The composite prepared by the experiment developed a pore structure and a crystal structure similar to SiO2 and chitosan chain-like coating was formed on the surface of fly ash. The new modified material has larger surface roughness, specific surface area and more adsorption channels. The Cr(VI) was enriched in modified materials by electrostatic adsorption between CrO42−, CrO72− and —NH3+ group and surface acid functional groups. The movement of Cr(VI) in solution is a diffusion process from the main body of the liquid phase to the surface of the liquid film.
摘要
以壳聚糖和粉煤灰为原料, 经乙酸和硫酸交联改性, 采用酸浸-包覆法制备壳聚糖包覆粉煤灰 (CWF)。利用扫描电子显微镜(SEM)和X 射线衍射(XRD)测试了CWF 的微观结构, 通过吸附实验研究 了CWF 对水中Cr(VI)的去除效果, 结合吸附模型, 扩散模型和动力学模型探讨了吸附机理。结果表 明: 实验制备的复合材料具有孔隙结构, 并在粉煤灰表面形成类似SiO2 和壳聚糖链状涂层的晶体结 构; 新型改性材料具有较大的表面粗糙度, 比表面积和更多的吸附通道; 改性材料通过CrO42−, Cr2O72−和—NH3+基团之间的静电吸引以及表面酸官能团的多组分吸附功能吸附Cr(VI); Cr(VI)在溶液中的运 动是液相主体向液膜表面扩散的过程。
Similar content being viewed by others
References
ZHANG Wei-fang, ZHANG Pei-xuan, LIU Feng, LIU Wei-zhen, ZHANG Jing, LIN Zhang. Simultaneous oxidation of Cr(III) and extraction of Cr(VI) from chromite ore processing residue by silicate-assisted hydrothermal treatment [J]. Chemical Engineering Journal, 2019, 371: 565–574. DOI: https://doi.org/10.1016/j.cej.2019.04.082.
AKAR S, LORESTANI B, SOBHANARDAKANI S, CHERAGHI M, MORADI O. Surveying the efficiency of Platanus orientalis bark as biosorbent for Ni and Cr(VI) removal from plating wastewater as a real sample [J]. Environmental Monitoring and Assessment, 2019, 191(6): 1–19. DOI: https://doi.org/10.1007/s10661-019-7479-z.
OUYANG Tong, TANG Ji-dan, LIU Fang, CHANG Chang-tang. Preparation of graphene oxide modified rice husk for Cr(VI) removal [J]. Journal of Nanoscience and Nanotechnology, 2019, 19(11): 7035–7043. DOI: https://doi.org/10.1166/jnn.2019.16662.
FILIK H, AVAN A A. Dextran modified magnetic nanoparticles based solid phase extraction coupled with linear sweep voltammetry for the speciation of Cr(VI) and Cr(III) in tea, coffee, and mineral water samples [J]. Food Chemistry, 2019, 292: 151–159. DOI: https://doi.org/10.1016/j.foodchem.2019.04.058.
LIU Lie, XU Yan, WANG Kun-peng, LI Kui-ling, XU Li-li, WANG Jian-bing, WANG Jun. Fabrication of a novel conductive ultrafiltration membrane and its application for electrochemical removal of hexavalent chromium [J]. Journal of Membrane Science, 2019, 584: 191–201. DOI: https://doi.org/10.1016/j.memsci.2019.05.018.
CHEN C H S, KUO T C, KUO H C, TSENG Y J, KUO C H, YUAN T H, CHAN Chang-chuan. Metabolomics of children and adolescents exposed to industrial carcinogenic pollutants [J]. Environmental Science & Technology, 2019, 53(9): 5454–5465. DOI: https://doi.org/10.1021/acs.est.9b00392.
LEGROURI K, KHOUYA E, HANNACHE H, EL H M, EZZINE M, NASLAIN R. Activated carbon from molasses efficiency for Cr(VI), Pb(II) and Cu(II) adsorption: A mechanistic study [J]. Chemistry International, 2017, 3(3): 301–310. DOI: https://doi.org/10.31221/osf.io/ecpm3.
LI Zi-han, XU Shu-yuan, XIAO Guang-hui, QIAN Li-min, SONG Yun. Removal of hexavalent chromium from groundwater using sodium alginate dispersed nano zero-valent iron [J]. Journal of Environmental Management, 2019, 244: 33–39. DOI: https://doi.org/10.1016/j.jenvman.2019.04.130.
SWAIDAN A, BORTHAKUR P, BORUAH P K, DAS M R, BARRAS A, HAMIEH S, TOUFAILY J, HAMIEH T, SZUNERITS S, BOUKHERROUB R. A facile preparation of CuS-BSA nanocomposite as enzyme mimics: Application for selective and sensitive sensing of Cr(VI) ions [J]. Sensors and Actuators B: Chemical, 2019, 294: 253–262. DOI: https://doi.org/10.1016/j.snb.2019.05.052.
MOBARAK M, MOHAMED E A, SELIM A Q, SELLAOUI L, LAMINE A, ERTO A, BONILLA-PETRICIOLET A, SELIEM M K. Surfactant-modified serpentine for fluoride and Cr(VI) adsorption in single and binary systems: Experimental studies and theoretical modeling [J]. Chemical Engineering Journal, 2019, 369: 333–343. DOI: https://doi.org/10.1016/j.cej.2019.03.086.
GAN Min, GU Chun-yao, DING Ji-juan, ZHU Jian-yu, LIU Xin-xing, QIU Guan-zhou. Hexavalent chromium remediation based on the synergistic effect between chemoautotrophic bacteria and sulfide minerals [J]. Ecotoxicology and Environmental Safety, 2019, 173: 118–130. DOI: https://doi.org/10.1016/j.ecoenv.2019.01.079.
BASHIR A, MALIK L A, AHAD S, MANZOOR T, BHAT M A, DAR G N, PANDITH A H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods [J]. Environmental Chemistry Letters, 2019, 17(2): 729–754. DOI: https://doi.org/10.1007/s10311-018-00828-y.
LIU Qun, XU Ming-jie, LI Feng, WU Tao, LI Yu-jiang. Rapid and effective removal of Cr(VI) from aqueous solutions using the FeCl3/NaBH4 system [J]. Chemical Engineering Journal, 2016, 296: 340–348. DOI: https://doi.org/10.1016/j.cej.2016.03.127.
CHENG Ce, HU Yong-you, SHAO Si-cheng, YU Jia-yuan, ZHOU Wei-jia, CHENG Jian-hua, CHEN Yuan-cai, CHEN Sheng-nan, CHEN Jun-feng, ZHANG Li-hua. Simultaneous Cr(VI) reduction and electricity generation in plant-sediment microbial fuel cells (P-SMFCs): Synthesis of non-bonding Co3O4 nanowires onto cathodes [J]. Environmental Pollution, 2019, 247: 647–657. DOI: https://doi.org/10.1016/j.envpol.2019.01.084.
SINGH D K, KUMAR V, MOHAN S, HASAN S H. Polylysine functionalized graphene aerogel for the enhanced removal of Cr(VI) through adsorption: Kinetic, isotherm, and thermodynamic modeling of the process [J]. Journal of Chemical and Engineering Data, 2017, 62(5): 1732–1742. DOI: https://doi.org/10.1021/acs.jced.7b00188.
KHAN M A, YUE Chun, FANG Zheng, HU Shuang-shuang, CHENG Hao, BAKRY A M, LIANG Li. Alginate/chitosan-coated zein nanoparticles for the delivery of resveratrol [J]. Journal of Food Engineering, 2019, 258: 45–53. DOI: https://doi.org/10.1016/j.jfoodeng.2019.04.010.
MOFARRAH A, HUSAIN T, CHEN Bing. Optimizing Cr(VI) adsorption on activated carbon produced from heavy oil fly ash [J]. Journal of Material Cycles and Waste Management, 2014, 16(3): 482–490. DOI: https://doi.org/10.1007/s10163-013-0197-7.
GONZÁLEZ A, MORENO N, NAVIA R, QUEROL X. Study of a Chilean petroleum coke fluidized bed combustion fly ash and its potential application in copper, lead and hexavalent chromium removal [J]. Fuel, 2010, 89(10): 3012–3021. DOI: https://doi.org/10.1016/j.fuel.2010.04.032.
RIBEIRO P B, de FREITAS V O, MACHRY K, MUNIZ A R C, da ROSA G S. Evaluation of the potential of coal fly ash produced by gasification as hexavalent chromium adsorbent [J]. Environmental Science and Pollution Research, 2019, 26(28): 28603–28613. DOI: https://doi.org/10.1007/s11356-018-3852-7.
ZHAO Sheng-xin, CHEN Zhong-lin, WANG Bin-yuan, SHEN Ji-min, ZHANG Jin-na, LI Dong-mei. Cr(VI) removal using different reducing agents combined with fly ash leachate: A comparative study of their efficiency and potential mechanisms [J]. Chemosphere, 2018, 213: 172–181. DOI: https://doi.org/10.1016/j.chemosphere.2018.08.143.
LIANG Hong-xu, SONG Bin, PENG Pai, JIAO Gao-jie, YAN Xiang, SHE Diao. Preparation of three-dimensional honeycomb carbon materials and their adsorption of Cr(VI) [J]. Chemical Engineering Journal, 2019, 367: 9–16. DOI: https://doi.org/10.1016/j.cej.2019.02.121.
AMINI F, SEMNANI D, KARBASI S, BANITABA S N. A novel bilayer drug-loaded wound dressing of PVDF and PHB/Chitosan nanofibers applicable for post-surgical ulcers [J]. International Journal of Polymeric Materials and Polymeric Biomaterials, 2019, 68(13): 772–777. DOI: https://doi.org/10.1080/00914037.2018.1506982.
RAJAMANI M, RAJENDRAKUMAR K. Chitosan-boehmite desiccant composite as a promising adsorbent towards heavy metal removal [J]. Journal of Environmental Management, 2019, 244: 257–264. DOI: https://doi.org/10.1016/j.jenvman.2019.05.056.
LIU Shan-shan, GE He-yi, CHENG Shi-lin, ZOU Yu, ZHANG Kai-li, CHU Cai-xia, GU Nian-liang. Synthesis of graphene oxide modified magnetic chitosan having skin-like morphology for methylene blue adsorption [J]. Journal of Nanoscience and Nanotechnology, 2019, 19(12): 7993–8003. DOI: https://doi.org/10.1166/jnn.2019.16872.
CHRISTOU C, PHILIPPOU K, KRASIA-CHRISTOFOROU T, PASHALIDIS I. Uranium adsorption by polyvinylpyrrolidone/chitosan blended nanofibers [J]. Carbohydrate Polymers, 2019, 219: 298–305. DOI: https://doi.org/10.1016/j.carbpol.2019.05.041.
World Health Organization. Guidelines for drinking-water quality [M]. IWA Publishing: 2011.
BAGHERI M, YOUNESI H, HAJATI S, BORGHEI S M. Application of chitosan-citric acid nanoparticles for removal of chromium (VI) [J]. International Journal of Biological Macromolecules, 2015, 80: 431–444. DOI: https://doi.org/10.1016/j.ijbiomac.2015.07.022.
DENG Xin, QI Li-qiang, ZHANG Ya-juan. Experimental study on adsorption of hexavalent chromium with microwave-assisted alkali modified fly ash [J]. Water, Air, & Soil Pollution, 2018, 229(1): 1–6. DOI: https://doi.org/10.1007/s11270-017-3679-8.
WANG Le-yang, XU Qi, ZHOU Qin, ZHANG Mei-yi, ZHAO Yuan. Adsorption and mechanism of phosphorus in water by lanthanum-aluminum/chitosan composite pellets [J]. Chinese Journal of Environmental Engineering, 2018, 12(9): 2490–2501. (in Chinese)
YANG Yong, ZHANG Peng-yu, JIANG Jin-long, DAI Yong, WU Mei, PAN Yi-chang, NI Ling-li. Synthesis and properties of magnetic zeolite with good magnetic stability from fly ash [J]. Journal of Sol-Gel Science and Technology, 2018, 87(2): 408–418. DOI: https://doi.org/10.1007/s10971-018-4733-8.
WEN Yue, TANG Zhi-ru, CHEN Yi, GU Yue-xia. Adsorption of Cr(VI) from aqueous solutions using chitosan-coated fly ash composite as biosorbent [J]. Chemical Engineering Journal, 2011, 175: 110–116. DOI: https://doi.org/10.1016/j.cej.2011.09.066.
VU D H, BUI H B, KALANTAR B, BUI X N, NGUYEN D A, LE Q T, DO N H, NGUYEN H. Composition and morphology characteristics of magnetic fractions of coal fly ash wastes processed in high-temperature exposure in thermal power plants [J]. Applied Sciences, 2019, 9(9): 1964. DOI: https://doi.org/10.3390/app9091964.
COPELLO G J, VARELA F, VIVOT R M, DÍAZ L E. Immobilized chitosan as biosorbent for the removal of Cd(II), Cr(III) and Cr(VI) from aqueous solutions [J]. Bioresource Technology, 2008, 99(14): 6538–6544. DOI: https://doi.org/10.1016/j.biortech.2007.11.055.
SHCHIPUNOV Y A. Sol-gel-derived biomaterials of silica and carrageenans [J]. Journal of Colloid and Interface Science, 2003, 268(1): 68–76. DOI: https://doi.org/10.1016/S0021-9797(03)00457-0.
HAN Cai-yun, YANG Ting, LIU Hang, YANG Liu, LUO Yong-ming. Characterizations and mechanisms for synthesis of chitosan-coated Na-X zeolite from fly ash and As(V) adsorption study [J]. Environmental Science and Pollution Research, 2019, 26(10): 10106–10116. DOI: https://doi.org/10.1007/s11356-019-04466-x.
YAN Min, BAI Yang, LI Shu-gang, LIN Hai-fei, YAN Dong-jie, SHU Chi-min. Factors influencing the gas adsorption thermodynamic characteristics of low-rank coal [J]. Fuel, 2019, 248: 117–126. DOI: https://doi.org/10.1016/j.fuel.2019.03.064.
WANG Ning, XU Zheng-he, XU Wei-ying, XU Jing, CHEN Ying-ying, ZHANG Min. Comparison of coagulation and magnetic chitosan nanoparticle adsorption on the removals of organic compound and coexisting humic acid: A case study with salicylic acid [J]. Chemical Engineering Journal, 2018, 347: 514–524. DOI: https://doi.org/10.1016/j.cej.2018.04.131.
WANG Shao-jie, BU Huai-tian, CHEN Huo-jun, HU Tian, CHEN Wen-zhao, WU Jin-hua, HU Han-jian, LIN Min-zhao, LI Yong-tao, JIANG Gang-biao. Floatable magnetic aerogel based on alkaline residue used for the convenient removal of heavy metals from wastewater [J]. Chemical Engineering Journal, 2020, 399: 125760. DOI: https://doi.org/10.1016/j.cej.2020.125760.
ANARAKDIM K, MATOS M, CAMBIELLA A, SENHADJI-KEBICHE O, GUTIÉRREZ G. Effect of temperature on the heat treatment to recover green solvent from emulsion liquid membranes used in the extraction of Cr(VI) [J]. Chemical Engineering and Processing-Process Intensification, 2020, 158: 108178. DOI: https://doi.org/10.1016/j.cep.2020.108178.
WU Bin, WANG Zi-ru, PENG Ding-hua, WANG Ying, HE Ting-ting, TANG Hao, XU Heng. Removal and recovery of heavy metals from soil with sodium alginate coated FeSSi nanocomposites in a leaching process [J]. Journal of Hazardous Materials, 2020, 398: 122732. DOI: https://doi.org/10.1016/j.jhazmat.2020.122732.
WU Yan, LUO Han-jin, WANG Hou, WANG Can, ZHANG Jian, ZHANG Zi-long. Adsorption of hexavalent chromium from aqueous solutions by graphene modified with cetyltrimethylammonium bromide [J]. Journal of Colloid and Interface Science, 2013, 394: 183–191. DOI: https://doi.org/10.1016/j.jcis.2012.11.049.
NOROUZI S, HEIDARI M, ALIPOUR V, RAHMANIAN O, FAZLZADEH M, MOHAMMADI-MOGHADAM F, NOURMORADI H, GOUDARZI B, DINDARLOO K. Preparation, characterization and Cr(VI) adsorption evaluation of NaOH-activated carbon produced from Date Press Cake; an agro-industrial waste [J]. Bioresource Technology, 2018, 258: 48–56. DOI: https://doi.org/10.1016/j.biortech.2018.02.106.
RAWAT A P, SINGH D P. Synergistic action of adsorption and reductive properties of ash derived from distilled Mentha piperita plant waste in removal of Cr(VI) from aqueous solution [J]. Ecotoxicology and Environmental Safety, 2019, 176: 27–33. DOI: https://doi.org/10.1016/j.ecoenv.2019.03.067.
MOGHADDAM A Z, ESMAEILKHANIAN E, SHAKOURIAN-FARD M. Immobilizing magnetic glutaraldehyde cross-linked chitosan on graphene oxide and nitrogen-doped graphene oxide as well-dispersible adsorbents for chromate removal from aqueous solutions [J]. International Journal of Biological Macromolecules, 2019, 128: 61–73. DOI: https://doi.org/10.1016/j.ijbiomac.2019.01.086.
ALAKHRAS F. Biosorption of Cd(II) ions from aqueous solution using chitosan-iso-vanillin as a low-cost sorbent: Equilibrium, kinetics, and thermodynamic studies [J]. Arabian Journal for Science and Engineering, 2019, 44(1): 279–288. DOI: https://doi.org/10.1007/s13369-018-3589-0.
TAN Guang-qun, LIU Yong, XIAO Dan. Preparation of manganese oxides coated porous carbon and its application for lead ion removal [J]. Carbohydrate Polymers, 2019, 219: 306–315. DOI: https://doi.org/10.1016/j.carbpol.2019.04.058.
PENG Rong-xin, CHEN Gui, ZHOU Fa, MAN Rui-lin, HUANG Jian-han. Catalyst-free synthesis of triazine-based porous organic polymers for Hg2+ adsorptive removal from aqueous solution [J]. Chemical Engineering Journal, 2019, 371: 260–266. DOI: https://doi.org/10.1016/j.cej.2019.04.063.
SONG Li, LIU Fu-qiang, ZHU Chang-qing, LI Ai-min. Facile one-step fabrication of carboxymethyl cellulose based hydrogel for highly efficient removal of Cr(VI) under mild acidic condition [J]. Chemical Engineering Journal, 2019, 369: 641–651. DOI: https://doi.org/10.1016/j.cej.2019.03.126.
DAVOODI S M, TAHERAN M, BRAR S K, GALVEZ-CLOUTIER R, MARTEL R. Hydrophobic dolomite sorbent for oil spill clean-ups: Kinetic modeling and isotherm study [J]. Fuel, 2019, 251: 57–72. DOI: https://doi.org/10.1016/j.fuel.2019.04.033.
LI S M S, ZENG Zuo-xiang, XUE Wei-lan. Adsorption of lead ion from aqueous solution by modified walnut shell: Kinetics and thermodynamics [J]. Environmental Technology, 2019, 40(14): 1810–1820. DOI: https://doi.org/10.1080/09593330.2018.1430172.
GÖÇENOĞLU SARiKAYA A. Kinetic and thermodynamic studies of the biosorption of Cr (VI) in aqueous solutions by Agaricus campestris [J]. Environmental Technology, 2021, 42(1): 72–80. DOI: https://doi.org/10.1080/09593330.2019.1620867.
SAAD A K, GOMES F P C, THOMPSON M R. Plasticizing effect of oxidized biodiesel on polyethylene observed by nondestructive method [J]. Fuel, 2019, 252: 246–253. DOI: https://doi.org/10.1016/j.foel.2019.04.122.
QIU Zhou-min, NIU Yu-zhong, FU Tian-tian, WANG Ke, MU Qiu-hong, WANG Feng. Removal of Ni(II) from fuel ethanol by PAMAM dendrimers/silica hybrid materials: Combined experimental and theoretical study [J]. Chemical Engineering Research and Design, 2019, 144: 174–184. DOI: https://doi.org/10.1016/j.cherd.2019.02.002.
KHALIFA R E, OMER A M, TAMER T M, SALEM W M, ELDIN M S M. Removal of methylene blue dye from synthetic aqueous solutions using novel phosphonate cellulose acetate membranes: Adsorption kinetic, equilibrium, and thermodynamic studies [J]. Desalination and Water Treatment, 2019, 144: 272–285. DOI: https://doi.org/10.5004/dwt.2019.23323.
ZHANG Rui-chi, LEIVISKÄ T, TANSKANEN J, GAO Baoyu, YUE Qin-yan. Utilization of ferric groundwater treatment residuals for inorganic-organic hybrid biosorbent preparation and its use for vanadium removal [J]. Chemical Engineering Journal, 2019, 361: 680–689. DOI: https://doi.org/10.1016/j.cej.2018.12.122.
LIANG Qian-wei, LUO Han-jin, GENG Jun-jie, CHEN Jing-da. Facile one-pot preparation of nitrogen-doped ultra-light graphene oxide aerogel and its prominent adsorption performance of Cr(VI) [J]. Chemical Engineering Journal, 2018, 338: 62–71. DOI: https://doi.org/10.1016/j.cej.2017.12.145.
SINGH R, DONG Hai-liang, ZENG Qiang, ZHANG Li, RENGASAMY K. Hexavalent chromium removal by chitosan modified-bioreduced nontronite [J]. Geochimica et Cosmochimica Acta, 2017, 210: 25–41. DOI: https://doi.org/10.1010/j.gca.2017.04.030.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item
Project(41602310) supported by the National Natural Science Foundation of China; Project(2017M611044) supported by the China Postdoctoral Science Foundation
Contributors
JIANG Chun-lu provided the funding acquisition, concept, and reviewed and edited the draft of manuscript. WANG Rui conducted the data curation and wrote the first draft of the manuscript. CHEN Xing conducted the literature review and wrote the first draft of the manuscript. ZHENG Liugen provided the methodology of the manuscript. CHENG Hua supervised the manuscript.
Conflict of interest
JIANG Chun-lu, WANG Rui, CHEN Xing, ZHENG Liu-gen, CHENG Hua declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jiang, Cl., Wang, R., Chen, X. et al. Preparation of chitosan modified fly ash under acid condition and its adsorption mechanism for Cr(VI) in water. J. Cent. South Univ. 28, 1652–1664 (2021). https://doi.org/10.1007/s11771-021-4724-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-021-4724-8