Abstract
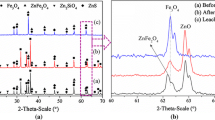
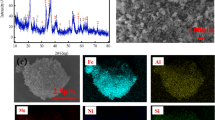
In this research work, extraction and purification of germanium from zinc leach residues (ZLR) were investigated. The results of ICP, XRF, and atomic adsorption spectroscopy (AAS) tests show that contents of germanium, iron, lead, and zinc within the leaching residue were 105×10−6, 3.53%, 10.35%, and 8.8%, respectively. XRD results indicate that the main minerals were in different forms of sulfates (CaSO4·2H2O, PbSO4 and ZnSO4·6H2O), silicate (SiO2), and oxide (Fe2O3). Dissolution of leaching filter cake was carried out using 5 parameters and each in 4 levels (acid concentration, temperature, time, liquid-to-solid ratio, and stirring speed) by Taguchi method (L16), and then optimization of the effective parameters by response surface method. Under optimum conditions, zinc and germanium dissolution efficiencies were 88.71% and 8%, respectively. Leaching tests with sulfuric acid (added di-ammonium oxalate monohydrate) and hydrochloric acid (HCl) on the residues obtained from previous-stage sulfuric acid dissolution, yielded germanium and iron recoveries of 83%, 88%, 40%, and 90%, respectively. Thus, leaching experiment with sulfuric acid (added di-ammonium oxalate monohydrate) was superior to that with hydrochloric acid due to high and low extraction amounts of germanium and iron, respectively. Precipitation experiments revealed that germanium purification with tannic acid presented a better result compared to sodium hydroxide and ammonia. Under optimum conditions, contents of germanium and iron in the solution after precipitation were 0.1505% and 14.7% with precipitation yields of 91% and 52%, respectively.
Similar content being viewed by others
References
ZHOU Z A, GUANG C H, GAN H X, YANG T Z, LIN C H. Ge and Cu recovery from precipitating vitriol supernatant in zinc plant [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(5): 1506–1511.
ZISCHKAU C, WOODBRIDGE N J. Separation of germanium: US, Patent 2249341 [P]. 1940.
LEBLEO A, FOSSI P, DEMARTHE J M. Process for the recovery and purification of germanium from zinc ore: US, Patent 4090871 [P]. 1978.
MENENDEZ F J S, MENENDEZ M S, CUADRA HERRERA A D L, TAMARGO F A, LORENZO L P, VALCARCEL M R, FERNANDEZ V A. Process for the recovery of germanium from solution that contain it: US, Patent 4886648 [P]. 1989.
TANG S F, ZHOU C S, JIANG X Y, ZHAO C L. Extraction separation of germanium with hydroxamic acid HGS98 [J]. Journal of Central South University of Technology, 2000, 7(1): 40–42.
LIANG D, WANG J, WANG Y. Difference in dissolution between germanium and zinc during the oxidative pressure leaching of sphalerite [J]. Hydrometallurgy, 2009, 95(1): 5–7.
LIANG J, FAN L, XU K, HUANG Y. Study on extracting of germanium with trioctylamine [J]. Energy Procedia, 2012, 17: 1965–1973.
SOYLAK M, YIGIT S. Preconcentration–separation of germanium at ultra trace levels on polysulfone membrane filter and its determination by spectrophotometry [J]. Journal of Industrial and Engineering Chemistry, 2015, 24: 322–325.
WOOD S A, SAMSON I M. The aqueous geochemistry of gallium, germanium, indium and scandium [J]. Ore Geology Reviews, 2006, 28(1): 57–102.
HE J, TANG M T, LIU Z Q, YANG S H, YAO W Y. Concentrating Ge in zinc hydrometallurgical process with hot acid leaching-halotrichite method [J]. Journal of Central South University of Technology, 2003, 10(4): 307–312.
HARBUCK D D. Gallium and germanium recovery from domestic sources [M]. Washington D. C. USA: US Department of the Interior, Bureau of Mines, 1992.
KUL M, TOPKAYA Y. Recovery of germanium and other valuable metals from zinc plant residues [J]. Hydrometallurgy, 2008, 92(3): 87–94.
DU F, LI J, LI X, ZHANG Z. Improvement of iron removal from silica sand using ultrasound-assisted oxalic acid [J]. Ultrasonics Sonochemistry, 2011, 18(1): 389–393.
NUSEN S, ZHU Z, CHAIRUANGSRI T, CHENG C Y. Recovery of germanium from synthetic leach solution of zinc refinery residues by synergistic solvent extraction using LIX 63 and Ionquest 801 [J]. Hydrometallurgy, 2015, 151: 122–132.
VIROLAINEN S, HEINONEN J, PAATERO E. Selective recovery of germanium with N-methylglucamine functional resin from sulfate solutions [J]. Separation and Purification Technology, 2013, 104: 193–199.
ARROYO F, FONT O, FERNÁNDEZ-PEREIRA C, QUEROL X, CHIMENOS J, ZEEGERS H. Germanium and gallium extraction from gasification fly ash: Optimisation for up-scaling a recovery process [C]// World of Coal Ash Conference. Lexington, KY, USA, 2009. http://www.flyash.info.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bayat, S., Aghazadeh, S., Noaparast, M. et al. Germanium separation and purification by leaching and precipitation. J. Cent. South Univ. 23, 2214–2222 (2016). https://doi.org/10.1007/s11771-016-3279-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-016-3279-6