Abstract
Purpose
Despite clear evidence-based supporting a benefit to exercise on physical and psychological metrics in patients with cancer, recruitment to exercise trials amongst cancer survivors is suboptimal. We explore current recruitment rates, strategies, and common barriers to participation in exercise oncology trials in cancer survivorship.
Methods
A systematic review was conducted using a pre-defined search strategy in EMBASE, CINAHL, Medline, Cochrane Library, and Web of Science. The search was performed up to 28/02/2022. Screening of titles and abstracts, full-text review, and data extraction was completed in duplicate.
Results
Of the 3204 identified studies, 87 papers corresponding to 86 trials were included. Recruitment rates were highly variable with a median rate of 38% (range 0.52–100%). Trials recruiting prostate cancer patients only had the highest median recruitment rate (45.9%) vs trials recruiting colorectal cancer patients only which had the lowest (31.25%). Active recruitment strategies such as direct recruitment via a healthcare professional were associated with higher recruitment rates (rho = 0.201, p = 0.064). Common reasons for non-participation included lack of interest (46.51%, n (number of studies) = 40); distance and transport (45.3%, n = 39); and failure to contact (44.2%, n = 38).
Conclusions
Recruitment of cancer survivors to exercise interventions is suboptimal with barriers being predominantly patient-oriented. This paper provides the benchmark for current recruitment rates to exercise oncology trials, providing data for trialists planning future trial design and implementation, optimise future recruitment strategies, and evaluate their own recruitment success against current practice.
Implications for Cancer Survivors
Enhanced recruitment to cancer survivorship exercise trials is necessary in facilitating the publication of definitive exercise guidelines, generalisable to varying cancer cohorts.
PROSPERO registration number
CRD42020185968.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Implementation of evidence-based healthcare is dependent upon stringent high-quality evidence from trials of robust methodological design, with inclusivity of representative patients, sufficient numbers to meet power calculations, and avoidance of bias. Large sample sizes are often required to minimise the risk of bias [1,2,3], produce data with narrow confidence intervals [4, 5], and enhance the statistical power [4,5,6] of a trial. The provision of such high-quality data may in turn underpin grade A recommendations that inform clinical practice [7, 8] and guidelines. Adequately powered trials assist with determining intervention efficacy, and external validity of results can be evaluated to a greater degree of certainty and guide the provision of evidence-based healthcare. Notwithstanding such major evident benefit of clinical trials, a bugbear of research is suboptimal recruitment of patients, with recruitment failure leading to the early termination of 19–40% of clinical trials [9]. Poor recruitment feeds into underpowered data and potential errors of interpretation with attendant risks of type II errors, wherein a null hypothesis is incorrectly accepted [6, 10]. These errors may lead to an over-exaggeration of treatment effect and fail to adequately assess associations with confounders that may impact intervention uptake [4, 11]. Moreover, a failure of trials to reach accrual targets results in increased costs and wasted resources [12, 13] and a failure of the fundamental objective to provide definitive conclusions informing practice [14]. Hence, trial recruitment represents a significant area of interest, in particular to ascertain the barriers and pitfalls that may be addressed.
An area of cancer research which has witnessed exponential growth in the past decade is that of exercise trials in cancer survivorship [14, 15]. The number of cancer survivors continues to increase globally due to advances in treatment options and increased early detection of cancers. In the USA alone, the American Cancer Society documented 16.9 million cancer survivors as of January 1st, 2019 [16]. Moreover, in the European context, ambitions exist to increase cancer survival beyond 10 years to 70% by 2035 [17]. However, whilst this trend is positive, there are now more and more people living with the significant health, social, and economic consequences of cancer and its treatments. The combined action of age-related physiological processes and pathological side effects related to cancer and its therapies has been shown to impact the long-term physical and psychological well-being of cancer survivors [18, 19]. These long-term effects including, but not limited to, cardiovascular disease [20,21,22,23], impaired physical function [24, 25], fatigue [26,27,28,29,30,31], and overall reduced quality of life [32,33,34] have prompted the movement towards the development of rehabilitative programmes in cancer survivorship [35,36,37,38] in order to enhance management of such symptoms and improve the overall quality of survivorship.
Exercise is a core rehabilitative intervention which has been utilised to varying levels of success to mitigate some of these negative sequelae of cancer and its treatments. As highlighted in the 2018 American College of Sports Medicine (ASCM) roundtable reports [14, 15], exercise has been shown to be a safe intervention for cancer survivors with strong evidence supporting its role in counteracting such cancer-related side effects as anxiety and depressive symptoms, fatigue, physical function, health-related quality of life, and lymphedema. With similar findings replicated following recent systematic review, the American Society of Clinical Oncology (ASCO) expert panel recommends that oncology healthcare providers endorse regular exercise by those patients undergoing active treatment [39]. Despite this, a number of knowledge gaps and biases still exist. There is, for instance, a predominance of exercise studies in cancer survivorship on common cancer types, namely, breast, prostate, and colorectal, with less on cancers such as lung, pancreas, and oesophageal [14, 15, 40], which can have a more significant physical toll. There is also arguably a bias towards inclusion of survivors with low or no disease burden and those with higher education status [14, 41, 42]. Accordingly, research conclusions may not be generalisable, and the direct application of current guidelines to alternate groups may not be valid [41]. A general gap also exist on the role of exercise on sleep [43,44,45,46], cognitive function [47, 48], pain [14, 49], and treatment tolerance [14, 50,51,52], in cancer patients. Importantly, optimal dosing for specific cancer types and patient sub-groups is absent, as trials directly comparing differing intervention prescriptions is minimal [14, 53]. Given this, the ASCM has suggested that future studies of adequate power and randomised controlled design be implemented in a variety of patient sub-groups, of varying prognoses, as to guide the development of exercise prescription in a manner similar to that seen in precision medicine [14].
A fundamental requirement for exercise trials in cancer survivorship is adequate recruitment. There has been an evident expansion of research in this field; however, recruitment of cancer patients to clinical trials has been shown to be less than 5% [54,55,56] with as little as 5.7% of these taking part in exercise interventions [57]. Continuing exercise oncology research in this way will waste valuable resources and slow the development and application of evidence-based rehabilitation for cancer survivors. As such, there is a clear need to gain greater insight into current recruitment practices used in exercise trials in cancer survivorship and to identify key barriers to participation for this specific cohort. As such, the aims of this systematic review are as follows:
-
i)
Examine current recruitment rates to exercise trials amongst cancer survivors.
-
ii)
Identify current recruitment strategies.
-
iii)
Identify common barriers to participation in exercise trials in cancer survivorship.
Methods
A systematic approach based on the PRISMA guidelines was applied in the reporting of this review. EMBASE, MEDLINE, CINAHL, Cochrane Library, and Web of Science were searched up until 28/02/2022. A search strategy was generated by the subject librarian (DM) using all keywords and subject headings included (Supplementary Material 1).
Eligibility criteria were (i) included adult cancer survivors, i.e. adult cancer patients with a histologically confirmed diagnosis of cancer of any type, who had completed primary treatment, e.g. surgery, neoadjuvant/adjuvant chemotherapy and/or radiotherapy; (ii) included a prescriptive exercise intervention; (iii) included a randomised controlled study design; (iv) included information regarding recruitment methods; and (vi) included information regarding recruitment rates. Exclusion criteria were (i) articles unavailable as full-text; (ii) articles unavailable in English; (iii) systematic reviews, meta-analysis, abstracts, conference proceedings, case studies, and letters to the editor; (iv) secondary articles, unless the original article failed to provide sufficient data (v) including participants under the age of 18 years of age; (vi) studies wherein recruitment rate is not provided nor calculable; and (vii) non-prescriptive exercise interventions or physical activity recommendations.
For the purpose of this review exercise was defined as “planned, structured and repetitive bodily movement, the objective of which is to improve or maintain physical fitness” [58]. This definition was inclusive of various exercise interventions including aerobic and resistance training, flexibility training such as yoga, and other physical activity programmes which followed a defined exercise prescription based on the frequency, intensity, type, and time (FITT) principle. Recruitment rate was defined as the percentage of potentially eligible participants that were recruited.
Utilising Covidence, an electronic systematic review management system, all titles and abstracts were reviewed by at least two independent reviewers from the review team (SR/LON/AM/AQK/AC). Articles not meeting the pre-defined inclusion criteria were excluded, with discrepancies resolved by a third independent reviewer (EG). The same process was applied to the subsequent full-text review. Details pertaining to author, year of publication, patient characteristics, recruitment rate, recruitment strategies, reasons for non-participation, and exercise interventions were extracted independently by SR, LON, LOC, AMcG, AQK, and AC. Where within text information regarding recruitment rate was insufficient, recruitment rates were calculated from information provided in the trials’ CONSORT flow diagram. Data was compared and inconsistencies resolved. Following extraction of recruitment methods and non-participation, findings were grouped according to common themes, with frequency expressed as a percentage of overall trials. In addition, the relationship between recruitment rates and method of recruitment was explored using Spearman rank order correlation in IBM SPSS. Assessment of risk of bias was not applicable to this systematic review as studies included were neither randomised nor blinded for the outcomes related to recruitment.
Results
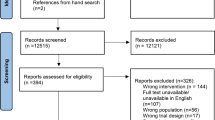
The literature search results are presented in Fig. 1. A total of 3204 studies were identified through the pre-defined search strategy completed on February 28th, 2022. Following removal of duplicates, a total of 1759 citations underwent abstract and title screening. One hundred eighty-one papers underwent full-text screening, of which 87 papers accounting for 86 trials were included in the final review. The full list and description of included studies are included as Supplemental Material 2. The full reference list and reason for exclusion of reports at full-text review stage are provided in Supplemental Material 3. Trials were published between 2003 and 2021. Over two thirds of trials were conducted in North America (n = 60, 69.77%) with the remainder from Europe (n = 9, 10.47%), Australia and New Zealand (n = 6, 6.98%), the UK and Northern Ireland (n = 5, 5.81%), Asia (n = 5, 5.81%), and South America (n = 1, 1.16%). Breast cancer survivors were the most commonly studied cohort, being the most prominent cancer type in 50 trials (58.1%), 36 including breast cancer only and 14 trials where breast cancer accounted for over 50% of study participants. This was followed by prostate cancer (n = 9, 10.5%), colorectal (n = 6, 6.98%), and gynaecological (n = 3, 3.5%), and 31 trials included a mix of cancer types (36.04%). Median intervention duration was 5 months (range 0.25–24), with a median follow-up of 6 months (range 0.25–24). Combined aerobic and resistance training accounted for over a third of interventions (n = 31, 36.05%), followed by physical activity (n = 18, 20.93%). Other interventions included aerobic training alone (n = 13, 15.17%), resistance training alone (n = 11, 12.79%), yoga (n = 7, 8.14%), walking (n = 5, 5.81%), Qigong (n = 2, 2.33%), Tai Chi (n = 1, 1.16%), line dancing (n = 1, 1.16%), and wall-climbing (n = 1, 1.16%). Seven trials (8%) involved a combined exercise and dietary intervention (dietary advice and/or diet supplementation).
Recruitment rates and methods to exercise trials for cancer survivors
Recruitment rates to individual trials are presented in Supplemental Material 2. The median recruitment rate for all trials was 38% (range 0.52–100%) (mean 42.96% (SD 27.2)). When categorised by cancer type (Table 1), trials recruiting prostate cancer patients only had the highest median recruitment rate of 45.9% (range 9.4–74%), followed by trials recruiting only breast cancer patients (median recruitment rate 44.4% (range 0.52–96.2%)), gynaecological cancer patients only (median recruitment rate 38.8% (range 21.1–90.91%)), trials with a mixed cancer population (median recruitment rate 36.6% (range 8–100%)), and those recruiting colorectal cancer patients only (median recruitment rate 31.25% (range 2.8–86.8%)).
Recruitment methods reported in included trials are described in Table 2 and include active recruitment strategies such as recruitment at medical or surgical clinics (n = 38, 44.2%), recruitment by healthcare professionals (n = 33, 38.4%), and advocacy groups or community events (n = 24, 27.9%) and passive recruitment strategies such as print materials or broadcast (n = 21, 24.4%), state cancer registries (n = 17, 19.8%), hospital (n = 16, 18.6%) or previous trial registries (n = 12, 13.7%), in-hospital advertisements (n = 10, 11.6%) and web-based advertisements (n = 10, 11.6%), and patient-led recruitment such as self-referral (n = 8, 9.3%) and word of mouth (n = 7, 8.1%). Correlation analysis showed that direct recruitment by medical professionals had a small but non-significant positive correlation with recruitment rate achieved (rho = 0.201, p = 0.064). Passive recruitment strategies such as invitation through state registries (rho = − 0.367, p = 0.001) and hospital registries (rho = − 0.255, p = 0.018) were negatively correlated with recruitment rates. No other significant correlations between recruitment method and rate were observed.
Reasons for non-participation in exercise trials for cancer survivors
Reasons for non-participation in exercise trials in cancer survivorship are outlined in Table 3. Over two thirds of trials failed to report specific reasons for non-participation (n = 50). The most common reasons for refusal to trial participation were distance and transport (n = 36, 48.6%), lack of interest (n = 35, 47.3%), failure to contact or no response (n = 34, 45.9%), time commitments (n = 31, 41.9%), and health concerns (n = 25, 33.8%). Other reasons were as follows: other commitments (n = 16, 21.6%), other reasons not specified (n = 16, 21.6%), lost to follow-up (n = 12, 16.2%), already exercising (n = 10, 13.5%), not willing to be randomised (n = 8, 10.8%), and not willing to complete outcome measures (n = 5, 6.8%).
Discussion
This is the first systematic review, to our knowledge, that provides a comprehensive overview of recruitment practices used in the accrual of cancer survivors to exercise interventions. Our findings reveal that recruitment rates to exercise interventional trials amongst cancer survivors are suboptimal (median 38%) in contrast to cancer clinical trials wherein a recruitment rate of 55% [59] is considered typical and highlight that current recruitment strategies are of varying efficacy.
This review reinforces previous findings on exercise rehabilitation in specific cancer types. Two recent systematic reviews examining the safety and feasibility of exercise amongst colorectal cancer patients [60] and stage II + breast cancer survivors [61] reported recruitment rates of 38% (range 4–91%) and 56% (range 1–96%), respectively. For those with advanced disease, two independent reviews noted a 49% accrual rate to exercise interventions to be typical [62, 63], whilst in a review of interventions promoting physical activity amongst cancer survivors, Turner et al. [64] noted varying recruitment rates between 9.5 and 95%. Furthermore, this systematic review is also the first to provide a breakdown of recruitment rates per cancer type in cancer survivorship. Significant variance in recruitment rates were observed between trials of different cancer types, with prostate cancer trials recording a median recruitment rate of 45.9%, but colorectal cancer trials only reaching a median recruitment target of 31.25%. Similar variability in recruitment rates is observed in exercise trials in non-cancer chronic diseases, including chronic obstructive pulmonary disease [65] and cardiovascular disease [66,67,68], with recruitment to pulmonary and cardiac rehabilitation varying from 35 to 100% and 4 to 100%, respectively. Accordingly, findings to date demonstrate an inherent variance in the degree of participation in exercise trials throughout healthcare but with the majority of studies recruiting less than 50%.
A further dimension of the recruitment problem is generalisability of data and results within a disease population [69,70,71]. For instance, the exclusion of ethnic and racial minorities [72,73,74], socioeconomically disadvantaged, and less educated cohorts [75,76,77] has increased over the last decade with studies demonstrating a disproportionate lack of awareness of clinical trial availability, despite equivalent willingness to participate [72, 78]. Patient barriers [72, 79, 80], trial design [79,80,81,82], and recruiter bias [79, 83] are amongst a few established factors limiting the inclusion of such patient groups. Similarly, as highlighted in this review, there is a marked predominance of specific cancer types, with 86.05% of included studies exclusively encompassing breast, prostate, and colorectal cancers, and moreover particularly focused on early-stage cancer survivors. These methodological errors may limit the external validity and broad generalisability, for instance, to other cancer types, patients with more advanced disease and particular social and racial demographics. A reasonable interpretation of the review data is that the majority of research falls short of the ACSM projection for precision exercise prescription [14] which requires the adaption of such exercise-based clinical trials as to include such minority groups in order to facilitate the development of a body of research that allows for individualised exercise prescriptions for cancer survivors.
If appropriate representation within trials is a fundamental to published trials, a more fundamental problem, perhaps easily addressed, is the methodology to recruit patients and the barriers that exist that may be circumvented. In this review, we have highlighted that recruitment of cancer survivors to exercise interventions is largely medically based, with correlation analysis suggesting a small but non-significant effect through direct recruitment by healthcare professional (HCP), with the converse appearing true for passive methods including the use of institutional databases. Greater access to the intended trial cohort by the healthcare team, a greater proportion of research-orientated clinicians and healthcare professionals, and trust established through patient–clinician relationships provide a significant opportunity for improved recruitment [84, 85]. However, for patients, misunderstanding of RCT concepts and design, discomfort with RCT eligibility criteria, lack of equipoise between treatment options, and difficulty exploring patient preferences are amongst a few clinician-related barriers which may impact recruitment [81, 86]. Although training has shown to improve recruiter confidence and communication [87], effects on trial accrual and patient satisfaction have been suboptimal [86, 87]. An intriguing suggestion to improve medically based recruitment is a prospective ‘trial by design’ [88], in which recruitment practices are evaluated and altered during the trials active phase. The QuinteT Recruitment Intervention is a similar concept: a two-phase intervention evaluating trial practices through reviews of trial documentation, audio-recordings of recruitment sessions, and interviews with recruitment staff and declining participants, which in turn guide revision of recruitment practices [89]. Such methods allow for alterations of recruitment design which address site- and cohort-specific barriers. Reporting such alterations in line with embedded recruitment trial guidelines [90] provides opportunity for accurate documentation of changes in recruitment strategies and direct comparison on recruitment effects.
It is clear from this review that the greatest barrier to participation in exercise trials in cancer survivorship are patient-centric, with transport and distance from intervention site, disinterest, time, commitments, and health concerns being commonly cited, consistent with previous reports [91,92,93,94,95,96]. Attempts made to date to implement strategies to enhance recruitment to clinical trials have largely not met their intended outcomes [97]. The absence of clarity as to why recruitment failure remains so prominent prompted the Prioritising Recruitment in Randomized Trials Priority Setting Partnership (PRioRiTy PSP), a collaborative effort aimed at identifying current recruitment uncertainties and prioritising the most relevant unanswered questions regarding recruitment [98]. This was done by way of steering groups and workshops inclusive of all research stakeholders from patients, recruiters, and those with research expertise and ultimately highlighted the need for greater public and patient involvement (PPI), defined as “research being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them” [99]. Consequently, PPI has become a central strategy in optimising recruitment to clinical trials [100,101,102], demonstrating modest but significant increases in recruitment rates when applied to areas of recruitment processes and trial design [103, 104]. Relating to cancer specifically, the use of PPI within clinical cancer trials has grown significantly, yet persistent issues regarding inclusivity of cohort representation remain [105]. Hence, the core problem that this initiative is designed to mitigate remains a concern. Furthermore, owing in part to poor standards of reporting, optimal PPI strategies are less clear [102, 106]. However, the recent expansion of study within a trial (SWAT) trials and the Trial Forge Initiative [107] within Ireland and the UK may aid in shedding some light on these current uncertainties and provide standardised, easily interpretable data which can guide future recruitment practices.
Limitations of review
We acknowledge some limitations. Although efforts were made to ensure all relevant papers were included, with a review of references and an additional hand search, it is possible that studies were missed or not included due to their unavailability in full-text form or in the English language. Furthermore, due to the heterogeneity of recruitment approaches, our review is unable to provide clear insight into definitive and effective recruitment measures or suggest optimal recruitment methods for variable cancer survivorship cohorts. Future studies should comparatively examine varying recruitment methods for cohorts of different cancer types and stages, disease, and socioeconomic burden. We also acknowledge that only RCTs including participants who had completed primary treatment prior to participation in an exercise intervention were included in this review. As the uptake of exercise interventions during active treatment increases, it would be useful to conduct a subsequent review into recruitment rates and strategies in that population.
Conclusion
Given the scientific and clinical evidence supporting the clear benefit of exercise on physical and mental well-being in cancer survivorship, recruitment strategies within such trials should be of a high priority in cancer research. This systematic review highlights that recruitment rates to exercise trials in cancer survivorship are most often suboptimal (median recruitment rate 38%), and greater utilisation of trials methodology research is required in such trials to improve patient recruitment and the quality of trials and the strength of the evidence and recommendations. The findings of this review provide trialists in exercise oncology research with a comprehensive overview of recruitment rates, strategies, and reasons for non-participation and should be invaluable in future planning of trials in this field.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Papageorgiou SN, Antonoglou GN, Tsiranidou E, Jepsen S, Jager A. Bias and small-study effects influence treatment effect estimates: a meta-epidemiological study in oral medicine. J Clin Epidemiol. 2014;67(9):984–92.
Chaimani A, Vasiliadis HS, Pandis N, Schmid CH, Welton NJ, Salanti G. Effects of study precision and risk of bias in networks of interventions: a network meta-epidemiological study. Int J Epidemiol. 2013;42(4):1120–31.
Lin L. Bias caused by sampling error in meta-analysis with small sample sizes. PLoS ONE. 2018;13(9):e0204056.
Rusticus SA, Lovate CY. Impact of sample size and variability on the power and type I error rates of equivalence tests: a simulation study. Practical Assessment, Research & Evaluation. 2014;19(11).
Jones SR, Carley S, Harrison M. An introduction to power and sample size estimation. Emerg Med J. 2003;20:453–8.
Biau DJ, Kerneis S, Porcher R. Statistics in brief: the importance of sample size in the planning and interpretation of medical research. Clin Orthop Relat Res. 2008;466(9):2282–8.
Jia B, Lynn HS. A sample size planning approach that considers both statistical significance and clinical significance. Trials. 2015;16:213.
Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990–6.
Carlisle B, Kimmelman J, Ramsay T, MacKinnon N. Unsuccessful trial accrual and human subjects protections: an empirical analysis of recently closed trials. Clin Trials. 2015;12(1):77–83.
Columb MO, Atkinson MS. Statistical analysis: sample size and power estimations. BJA Education. 2016;16(5):159–61.
Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ. 2013;346:f2304.
Kitterman DR, Cheng SK, Dilts DM, Orwoll ES. The prevalence and economic impact of low-enrolling clinical studies at an academic medical center. Acad Med. 2011;86(11):1360–6.
Kakumanu S, Manns BJ, Tran S, Saunders-Smith T, Hemmelgarn BR, Tonelli M, et al. Cost analysis and efficacy of recruitment strategies used in a large pragmatic community-based clinical trial targeting low-income seniors: a comparative descriptive analysis. Trials. 2019;20(1):577.
Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–90.
Patel AV, Friedenreich CM, Moore SC, Hayes SC, Silver JK, Campbell KL, et al. American College of Sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019;51(11):2391–402.
Society AC. Cancer facts & firgures 2020. Atlanta: American Cancer Society; 2020.
Lawler M, Oliver K, Gijssels S, Aapro M, Abolina A, Albreht T, et al. The European Code of Cancer Practice. J Cancer Policy. 2021;28: 100282.
Shi Q, Smith TG, Michonski JD, Stein KD, Kaw C, Cleeland CS. Symptom burden in cancer survivors 1 year after diagnosis: a report from the American Cancer Society’s Studies of Cancer Survivors. Cancer. 2011;117(12):2779–90.
Bubis LD, Davis L, Mahar A, Barbera L, Li Q, Moody L, et al. Symptom burden in the first year after cancer diagnosis: an analysis of patient-reported outcomes. Journal of Clinical Oncology. 2018;36.
Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016;66(4):309–25.
Bradshaw PT, Stevens J, Khankari N, Teitelbaum SL, Neugut AI, Gammon MD. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27(1):6–13.
Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. The Lancet. 2019;394(10203):1041–54.
Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J. 2017;93(1096):82–90.
Naughton MJ, Weaver KE. Physical and mental health among cancer survivors: considerations for long-term care and quality of life. North Caroline Med J. 2014;75(4):283–6.
Petrick JL, Reeve BB, Kucharska-Newton AM, Foraker RE, Platz EA, Stearns SC, et al. Functional status declines among cancer survivors: trajectory and contributing factors. J Geriatr Oncol. 2014;5(4):359–67.
Horneber M, Fischer I, Dimeo F, Ruffer JU, Weis J. Cancer-related fatigue: epidemiology, pathogenesis, diagnosis, and treatment. Dtsch Arztebl Int. 2012;109(9):161–71; quiz 72.
Fabi A, Falcicchio C, Giannarelli D, Maggi G, Cognetti F, Pugliese P. The course of cancer related fatigue up to ten years in early breast cancer patients: what impact in clinical practice? Breast. 2017;34:44–52.
Ruiz-Casado A, Alvarez-Bustos A, de Pedro CG, Mendez-Otero M, Romero-Elias M. Cancer-related fatigue in breast cancer survivors: a review. Clin Breast Cancer. 2020.
Abrahams HJG, Gielissen MFM, Schmits IC, Verhagen C, Rovers MM, Knoop H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: a meta-analysis involving 12 327 breast cancer survivors. Ann Oncol. 2016;27(6):965–74.
Jones JM, Olson K, Catton P, Catton CN, Fleshner NE, Krzyzanowska MK, et al. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2016;10(1):51–61.
Cleeland CS, Zhao F, Chang VT, Sloan JA, O’Mara AM, Gilman PB, et al. The symptom burden of cancer: evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer. 2013;119(24):4333–40.
Firkins J, Hansen L, Driessnack M, Dieckmann N. Quality of life in “chronic” cancer survivors: a meta-analysis. J Cancer Surviv. 2020;14(4):504–17.
Arndt V, Koch-Gallenkamp L, Jansen L, Bertram H, Eberle A, Holleczek B, et al. Quality of life in long-term and very long-term cancer survivors versus population controls in Germany. Acta Oncol. 2017;56(2):190–7.
Wu HS, Harden JK. Symptom burden and quality of life in survivorship: a review of the literature. Cancer Nurs. 2015;38(1):E29-54.
Santa Mina D, Fong AJ, Petrella AR, Culos-Reed SN, Chasen M, Sabiston CM. Moving research into practice: summary report of the Ex/Cancer meeting on physical activity, exercise, and rehabilitation in oncology. Current Oncology. 2018;25(6).
Scott DA, Mills M, Black A, Cantwell M, Campbell A, Cardwell CR, et al. Multidimensional rehabilitation programmes for adult cancer survivors. Cochrane Database Syst Rev. 2013;3:007730.
Smith SR, Zheng JY, Silver J, Haig AJ, Cheville A. Cancer rehabilitation as an essential component of quality care and survivorship from an international perspective. Disabil Rehabil. 2020;42(1):8–13.
Lukez A, Baima J. The role and scope of prehabilitation in cancer care. Semin Oncol Nurs. 2020;36(1):150976.
J.A. L, K. B, A.M. M, S.K. C, W. D-W, S.C G, et al. Exercise, diet, and weight management during cancer treatment: ASCO Guideline. Journal of Clinical Oncology. 2022.
McTiernan A, Friedenreich CM, Katzmarzyk PT, Powell KE, Macko R, Buchner D, et al. Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc. 2019;51(6):1252–61.
Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495.
Bower P, Grigoroglou C, Anselmi L, Kontopantelis E, Sutton M, Ashworth M, et al. Is health research undertaken where the burden of disease is greatest? Observational study of geographical inequalities in recruitment to research in England 2013–2018. BMC Med. 2020;18(1):133.
Mercier J, Savard J, Bernard P. Exercise interventions to improve sleep in cancer patients: a systematic review and meta-analysis. Sleep Med Rev. 2017;36:43–56.
Hidde MC, Leach HJ, Marker RJ, Peters JC, Purcell WT. Effects of a clinic-based exercise program on sleep disturbance among cancer survivors. Integr Cancer Ther. 2020;19:1–8.
Kreutz C, Schmidt ME, Steindorf K. Effects of physical and mind-body exercise on sleep problems during and after breast cancer treatment: a systematic review and meta-analysis. Breast Cancer Res Treat. 2019;176(1):1–15.
Howell D, Oliver TK, Keller-Olaman S, Davidson JR, Garland S, Samuels C, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol. 2014;25(4):791–800.
Zimmer P, Baumann FT, Oberste M, Wright P, Garthe A, Schenk A, et al. Effects of exercise interventions and physical activity behavior on cancer related cognitive impairments: a systematic review. Biomed Res Int. 2016;2016:1820954.
Myers JS, Erickson KI, Sereika SM, Bender CM. Exercise as an intervention to mitigate decreased cognitive function from cancer and cancer treatment: an integrative review. Cancer Nurs. 2018;41(4):327–43.
Griffith K, Wenzel J, Shang J, Thompson C, Stewart K, Mock V. Impact of a walking intervention on cardiorespiratory fitness, self-reported physical function, and pain in patients undergoing treatment for solid tumors. Cancer. 2009;115(20):4874–84.
Bland KA, Zadravec K, Landry T, Weller S, Meyers L, Campbell KL. Impact of exercise on chemotherapy completion rate: a systematic review of the evidence and recommendations for future exercise oncology research. Crit Rev Oncol Hematol. 2019;136:79–85.
Kirkham AA, Gelmon KA, Van Patten CL, Bland KA, Wollmann H, McKenzie DC, et al. Impact of exercise on chemotherapy tolerance and survival in early-stage breast cancer: a nonrandomized controlled trial. J Natl Compr Canc Netw. 2020;18(12):1670–7.
Mijwel S, Bolam KA, Gerrevall J, Foukakis T, Wengstrom Y, Rundqvist H. Effects of exercise on chemotherapy completion and hospitalization rates: the OptiTrain Breast Cancer Trial. Oncologist. 2020;25(1):23–32.
Schumann M, Freitag N, Bloch W. Advanced exercise prescription for cancer patients and its application in Germany. Journal of Science in Sport and Exercise. 2020;2(3):201–14.
Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. J Am Med Assoc. 2004;291(22):2720–6.
National Cancer Strategy 2017–2026. 2017.
Trials ENtACC. Five steps to emhance patient participation in cancer clinical trials: guide and workbook. 2011.
Adams RN, Mosher CE, Blair CK, Snyder DC, Sloane R, Demark-Wahnefried W. Cancer survivors’ uptake and adherence in diet and exercise intervention trials: an integrative data analysis. Cancer. 2015;121(1):77–83.
Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical health: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31.
Unger JM, Hershman DL, Till C, Minasian LM, Osarogiagbon RU, Fleury ME, et al. 2020 “When Offered to Participate”: a systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. JNCI Journal of the National Cancer Institute 113(3):244–57
Singh B, Hayes SC, Spence RR, Steele ML, Millet GY, Gergele L. Exercise and colorectal cancer: a systematic review and meta-analysis of exercise safety, feasibility and effectiveness. Int J Behav Nutr Phys Act. 2020;17(1):122.
Singh B, Spence RR, Steele ML, Sandler CX, Peake JM, Hayes SC. A systematic review and meta-analysis of the safety, feasibility, and effect of exercise in women with stage II+ breast cancer. Arch Phys Med Rehabil. 2018;99(12):2621–36.
Sheill G, Guinan E, Brady L, Hevey D, Hussey J. Exercise interventions for patients with advanced cancer: a systematic review of recruitment, attrition, and exercise adherence rates. Palliat Support Care. 2019;17(6):686–96.
Dittus KL, Gramling RE, Ades PA. Exercise interventions for individuals with advanced cancer: a systematic review. Prev Med. 2017;104:124–32.
Turner RR, Steed L, Quirk H, Greasley RU, Saxton JM, Taylor SJ, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2018;9:010192.
Sohanpal R, Hooper R, Hames R, Priebe S, Taylor S. Reporting participation rates in studies of non-pharmacological interventions for patients with chronic obstructive pulmonary disease: a systematic review. BioMed Central. 2012;1(66).
Benzer W, Rauch B, Schmid JP, Zwisler AD, Dendale P, Davos CH, et al. Exercise-based cardiac rehabilitation in twelve European countries results of the European cardiac rehabilitation registry. Int J Cardiol. 2017;228:58–67.
Bjarnason-Wehrens B, McGee H, Zwisler AD, Piepoli MF, Benzer W, Schmid JP, et al. Cardiac rehabilitation in Europe: results from the European Cardiac Rehabilitation Inventory Survey. Eur J Cardiovasc Prev Rehabil. 2010;17(4):410–8.
Sumner J, Grace SL, Doherty P. Predictors of cardiac rehabilitation utilization in England: results from the national audit. J Am Heart Assoc. 2016;5(10).
Rothwell PM. External validity of randomised controlled trials: “To whom do the results of this trial apply?” The Lancet. 2005;365(9453):82–93.
Avellar SA, Thomas J, Kleinman R, Sama-Miller E, Woodruff SE, Coughlin R, et al. External validity: the next step for systematic reviews? Eval Rev. 2017;41(4):283–325.
Bonell C, Oakley A, Hargreaves J, Strange V, Rees R. 2006 Assessment of generalisability in trials of health interventions: suggested framework and systematic review. British Medical Journal 333(346–349)
Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23(4):327–37.
Duma N, Aguilera JV, Paludo J, Haddox CL, Valez MG, Want Y, et al. Representation of minorities and women in oncology clinical trials: review of the past 14 years. American Society of Clinical Oncology. 2018;14(1).
Godden S, Ambler G, Pollock AM. Recruitment of minority ethnic groups into clinical cancer research trials to assess adherence to the principles of the Department of Health Research Governance Framework: national sources of data and general issues arising from a study in one hospital trust in England. J Med Ethics. 2010;36(6):358–62.
Mohd Noor A, Sarker D, Vizor S, McLennan B, Hunter S, Suder A, et al. Effect of patient socioeconomic status on access to early-phase cancer trials. J Clin Oncol. 2013;31(2):224–30.
Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684–7.
Baquet CR, Commiskey P, Daniel Mullins C, Mishra SI. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detect Prev. 2006;30(1):24–33.
Trant AA, Walz L, Allen W, DeJesus J, Hatzis C, Silber A. Increasing accrual of minority patients in breast cancer clinical trials. Breast Cancer Res Treat. 2020;184(2):499–505.
Hussain-Gambles M, Atkin K, Leese B. Why ethnic minority groups are under-represented in clinical trials: a review of the literature. Health Soc Care Community. 2004;12:382–8.
Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–42.
Elliott D, Husbands S, Hamdy FC, Holmberg L, Donovan JL. Understanding and improving recruitment to randomised controlled trials: qualitative research approaches. Eur Urol. 2017;72(5):789–98.
Karim S, Xu Y, Kong S, Abdel-Rahman O, Quan ML, Cheung WY. Generalisability of common oncology clinical trial eligibility criteria in the real world. Clin Oncol (R Coll Radiol). 2019;31(9):e160–6.
Niranjan SJ, Martin MY, Fouad MN, Vickers SM, Wenzel JA, Cook ED, et al. Bias and stereotyping among research and clinical professionals: perspectives on minority recruitment for oncology clinical trials. Cancer. 2020;126(9):1958–68.
R.L. C, J.D. M, D.D. C, L.G. K. Physician-related factors involved in patient decisions to enroll onto cancer clinical trials. Journal of Oncology Practice. 2009;5(2).
Comis RL, Miller JD, Colaizzi DD, Kimmel LG. Physician-related factors involved in patient decisions to enroll onto cancer clinical trials. Journal of Oncology Practice. 2009;5(2).
Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open. 2012;2(1):e000496.
Townsend D, Mills N, Savovic J, Donovan JL. A systematic review of training programmes for recruiters to randomised controlled trials. Trials. 2015;16:432.
Meeker-O’Connell A, Glessner C, Behm M, Mulinde J, Roach N, Sweeney F, et al. Enhancing clinical evidence by proactively building quality into clinical trials. Clin Trials. 2016;13(4):439–44.
Rooshenas L, Scott LJ, Blazeby JM, Rogers CA, Tilling KM, Husbands S, et al. The QuinteT Recruitment Intervention supported five randomized trials to recruit to target: a mixed-methods evaluation. J Clin Epidemiol. 2019;106:108–20.
Madurasinghe VW, Sandra Eldridge on behalf of MRCSG, Gordon Forbes on behalf of the SECG. Guidelines for reporting embedded recruitment trials. Trials. 2016;17:27.
Walsh E, Sheridan A. Factors affecting patient participation in clinical trials in Ireland: a narrative review. Contemp Clin Trials Commun. 2016;3:23–31.
Mills EJ, Seely D, Rachlis B, Griffith L, Wu P, Wilson K, et al. Barriers to participation in clinical trials of cancer: a meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7(2):141–8.
Hardcastle SJ, Maxwell-Smith C, Kamarova S, Lamb S, Millar L, Cohen PA. Factors influencing non-participation in an exercise program and attitudes towards physical activity amongst cancer survivors. Support Care Cancer. 2018;26(4):1289–95.
Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143–56.
Avis NE, Smith KW, Link CL, Hortobagyi GN, Rivera E. Factors associated with participation in breast cancer treatment clinical trials. J Clin Oncol. 2006;24(12):1860–7.
Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psychooncology. 2018;27(3):713–24.
Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev. 2018;2:000013.
Healy P, Galvin S, Williamson PR, Treweek S, Whiting C, Maeso B, et al. Identifying trial recruitment uncertainties using a James Lind Alliance Priority Setting Partnership - the PRioRiTy (Prioritising Recruitment in Randomised Trials) study. Trials. 2018;19(1):147.
INVOLVE. : what is public involvement in research? 2015 [Available from: https://www.invo.org.uk/find-out-more/what-is-public-involvement-in-research-2/.
Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. 2014;17(5):637–50.
Baldwin JN, Napier S, Neville S, Wright-St Clair VA. Impacts of older people’s patient and public involvement in health and social care research: a systematic review. Age Ageing. 2018;47(6):801–9.
Sacristan JA, Aguaron A, Avendano-Sola C, Garrido P, Carrion J, Gutierrez A, et al. Patient involvement in clinical research: why, when, and how. Patient Prefer Adherence. 2016;10:631–40.
Crocker JC, Ricci-Cabello I, Parker A, Hirst JA, Chant A, Petit-Zeman S, et al. Impact of patient and public involvement on enrolment and retention in clinical trials: systematic review and meta-analysis. BMJ. 2018;363:k4738.
Price A, Albarqouni L, Kirkpatrick J, Clarke M, Liew SM, Roberts N, et al. Patient and public involvement in the design of clinical trials: an overview of systematic reviews. J Eval Clin Pract. 2018;24(1):240–53.
Pii KH, Schou LH, Piil K, Jarden M. Current trends in patient and public involvement in cancer research: a systematic review. Health Expect. 2019;22(1):3–20.
Greenhalgh T, Hinton L, Finlay T, Macfarlane A, Fahy N, Clyde B, et al. Frameworks for supporting patient and public involvement in research: systematic review and co-design pilot. Health Expect. 2019;22(4):785–801.
Treweek S, Bevan S, Bower P, Campbell M, Christie J, Clarke M, et al. Trial forge guidance 1: what is a Study within a trial (SWAT)? Trials. 2018;19(1):139.
Acknowledgements
The study team would like to acknowledge the support of Professor Martina Hennessy, the Health Research Trials Methodology Research Network (HRB-TMRN) lead at Trinity College Dublin, the University of Dublin, during the time of this project.
Funding
Open Access funding provided by the IReL Consortium Author SAR’s time was funded by a Health Research Trials Methodology Research Network (HRB-TMRN) 2020 Summer Scholarship. Author’s LOC and LON are funded by the Health Research Board (HRB-DIFA-FA-2018–009).
Author information
Authors and Affiliations
Contributions
LON conceived this study and decided on the framework for analysis. DM devised the search strategy. SAR, LOC, AMcG, AQK, AC, EG, and LON carried out the literature search and analysis. SAR drafted the manuscript. All authors have read and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reynolds, S.A., O’Connor, L., McGee, A. et al. Recruitment rates and strategies in exercise trials in cancer survivorship: a systematic review. J Cancer Surviv (2023). https://doi.org/10.1007/s11764-023-01363-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11764-023-01363-8