Abstract
Purpose
Advances in treatment, including biological and precision therapies, mean that more people are living with advanced cancer. Supportive care needs likely change across the cancer journey. We systematically identified instruments available to assess unmet needs of advanced cancer patients and evaluated their development, content, and quality.
Methods
Systematic searches of MEDLINE, CINAHL, Embase, PubMed, and PsycINFO were performed from inception to 11 January 2021. Independent reviewers screened for eligibility. Data was abstracted on instrument characteristics, development, and content. Quality appraisal included methodological and quality assessment, GRADE, feasibility, and interpretability, following consensus-based standards for the selection of health measurement instruments (COSMIN) guidelines.
Results
Thirty studies reporting 24 instruments were identified. These were developed for general palliative patients (n = 2 instruments), advanced cancer (n = 8), and cancer irrespective of stage (n = 14). None focused on patients using biological or precision therapies. The most common item generation and reduction techniques were amending an existing instrument (n = 11 instruments) and factor analysis (n = 8), respectively. All instruments mapped to ≥ 5 of 11 unmet need dimensions, with Problems and Needs in Palliative Care (PNPC) and Psychosocial Needs Inventory (PNI) covering all 11. No instrument reported all of the COSMIN measurement properties, and methodological quality was variable.
Conclusions
Many instruments are available to assess unmet needs in advanced cancer. There is extensive heterogeneity in their development, content, and quality.
Implications for Cancer Survivors
Given the growth of precision and biological therapies, research needs to explore how these instruments perform in capturing the needs of people using such therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For many cancers, survival continues to improve [1, 2]. This progress is, in part, due to medical advancements, such as improved diagnostic techniques and more effective treatment strategies – examples of which include precision and biological therapies [3, 4]. These agents are primarily – although not exclusively – used for patients with advanced disease. While they may improve survival, they are associated with adverse effects (e.g. cardiac dysfunction, hypertension, and skin rashes [5, 6]), which are different to those associated with traditional chemotherapeutic treatments. Patients using these new therapies may therefore experience a different symptom burden. Changes in advanced cancer prognosis, largely due to the growth in these therapies, mean that the cancer experience, once typified by rapid progression, may now be a prolonged and uncertain illness trajectory [7].
Previous work has identified a comprehensive range of unmet supportive care needs among advanced cancer patients [8, 9]. The most common dimensions of unmet need are physical, psychological, informational, and functional, with others including social, activities of daily living, healthcare, spiritual, sexual, and economic also described. People with early or advanced cancer may experience similar areas of unmet need. However, the prevalence, severity, and relative distribution of needs may vary between the two patient groups. This could result from location of the cancer (e.g. metastatic disease may result in more pain and greater physical unmet needs), different treatment (e.g. the differing adverse effect profiles of biological and precision therapies, compared to traditional chemotherapy), and prognosis (e.g. psychological unmet needs among patients with advanced disease may be dominated by emotions and worries about coming to the end of life). In terms of consequences, unmet needs in people with advanced cancer have been associated with more symptom distress, greater anxiety, and reduced quality of life [9]. Supportive care that is not consistent with patient needs could be detrimental to the patient, their caregiver, and even healthcare expenditure [10, 11].
Despite many examples of needs assessment tools and instruments available for use in people with cancer [12], it is not known which, if any, specifically capture the needs of people with advanced cancer. As supportive care needs change across the cancer journey [13], establishing what instruments are available to assess the needs of people with advanced cancer must be understood. This is particularly important when you consider the unique challenges presented by the new biological and precision therapies in this context. Our systematic review, therefore, aimed to address this question and (1) examine what instruments are available to measure unmet needs in people with advanced cancer and (2) assess instrument development, content, and quality, in terms of clinimetric properties.
Methods
This systematic review was registered with the Prospective Register for Systematic Reviews (PROSPERO) (CRD42020169278) and conducted and reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [14].
Definitions
There are multiple ways in which ‘advanced cancer’ can be defined. For the purposes of this systematic review, advanced disease was considered to be patients with stage IV, metastatic or incurable disease, or those undergoing palliative care.
An ‘unmet need’ was defined as something that a patient experiences as a problem and which they would like help or support with.
Eligibility criteria
A study was eligible if: (1) it reported on the development and/or validation of an instrument to measure unmet needs; (2) it included or signposted to the instrument items; (3) advanced cancer patients were included in the development or validation of the instrument; and (4) it was an original article, available in English. The instrument that a study pertains to was eligible if: (1) it was developed for cancer or palliative patients; (2) it measured more than one dimension of unmet need; and (3) it was available in English.
A study was excluded if: (1) the instrument was targeted at childhood/adolescent cancer patients, or survivors of cancer diagnosed in childhood/adolescence; (2) the authors did not report any validation for the instrument; (3) the patient was not the respondent; and (4) ≥ 50% of the instrument items and response options did not either allow patients to indicate a desire for help or support or use terminology that could infer a need/desire for help or support.
Search strategy
Five electronic databases were searched from inception: MEDLINE, Embase, PsycINFO, CINAHL, and PubMed. The search strategy concerned four key concepts (cancer, advanced disease, needs, and instrument), and was undertaken in March 2020. A combination of medical subject headings and keywords was formulated, with assistance from a senior library assistant (Online Resource 1), and informed by previously published search strategies [8]. Searches were tailored in accordance with the specific subject headings within each database (Online Resource 2).
The reference lists and forward citations of eligible studies and relevant systematic reviews were handsearched to identify additional studies. The search was updated on 11 January 2021, with no new studies identified.
Study selection
After duplicate studies were removed, titles and abstracts and then full-texts of potentially eligible studies were independently screened by two researchers (BR and LC). Disagreements were resolved through discussion and consensus with other authors, if required. Where cancer stage of study participants was not reported, authors were contacted to confirm inclusion of advanced cancer patients in the instrument’s development or validation. If eligibility was not confirmed, the instrument was excluded. If an existing instrument was adapted or shortened, with separate validation, this was included as a separate instrument. If an existing instrument was refined (e.g. item wording was modified), only the refined version was included.
Data extraction
General characteristics of included instruments
Data extraction was undertaken by BR, following a structured data extraction form, and checked by LS and AT. Extracted data included instrument name; purpose; target population; validations in languages other than English; study setting; study population; number of questions; mode of administration; recall period; time to complete; scoring; response options; item generation (e.g. patient interviews) and reduction (e.g. item response frequencies); and unmet need dimensions measured. Disagreements were resolved through discussion and consensus.
Published papers reporting additional development and/or validation studies relating to the included instruments were identified and used in data extraction where relevant. Where multiple papers were available for an instrument, characteristics of the study population were extracted from the initial (first published) validation study; all relevant papers were used in the final instrument development and content, and findings were pooled across papers for assessment of clinimetric properties.
Content analysis
Instrument content was mapped against nine previously identified dimensions of unmet need [8, 9]. Two additional dimensions, autonomy and role, were added based on the content of the eligible instruments (Online Resource 3). Any content that did not map onto these dimensions was reported as ‘other’.
Instrument items were included in this mapping if the items and/or response options allowed respondents to indicate a desire for help or difficulty with the item, thus signifying or inferring an unmet need. Dimension development was categorised as statistical (e.g. factor analysis), literature, conceptual, or other.
Clinimetric properties
The COSMIN checklist [15, 16] (Online Resource 4) was used to assess the methodological quality of the included instruments. COSMIN evaluates the development, validity, reliability, and responsiveness of instruments. The checklist is divided into ten measurement properties: development, content validity, structural validity, hypotheses testing, internal consistency, reliability, measurement error, cross-cultural validity, criterion validity, and responsiveness. Cross-cultural validity was not measured in this review because clinimetrics were only assessed in the original English language versions of the instruments. Criterion validity was omitted because there is no gold standard for needs assessments, due to the subjective nature of perceived needs [17].
Methodological quality assessment
The evaluation of each clinimetric property comprised 3–35 items. Each item is rated on a four-point scale: very good, adequate, doubtful, or inadequate. In accordance with ‘the worst score counts’ principle of COSMIN [15], the lowest score within a measurement property determined the methodological quality rating given to the instrument for that property.
GRADE was used to summarise the quality of available evidence. This concerned risk of bias, inconsistency, imprecision, and indirectness. Evidence was downgraded appropriately (Online Resource 5) and could be high (†††), moderate (††), low (†), or very low (-) in quality.
Quality criteria for the measurement properties
The quality of six measurement properties was assessed using a three-point scale: sufficient (+), insufficient (-), and indeterminate (?). This applied to structural validity, internal consistency, reliability, measurement error, hypotheses testing for construct validity, and responsiveness.
Feasibility and interpretability
To inform usability, instrument feasibility and interpretability were extracted. Feasibility within COSMIN concerns the ease of applying the instrument in its intended context of use, so aspects including instrument length, completion time, and type and ease of administration were extracted. Interpretability concerns the extent to which meaning can be assigned to quantitative scores, so aspects including distribution of scores and percentage of missing items and missing total scores were extracted.
Results
Search results
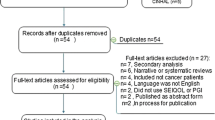
The database searches identified 4991 hits, with 2794 remaining after deduplication. After title and abstract screening, 130 full-text articles were assessed for eligibility, and of these, 13 studies were eligible. Following hand searching, an additional 17 studies were identified and deemed eligible. Overall, 30 papers reporting on 24 unique instruments were included in the review (Fig. 1).
Study population for validation
Of the 24 instruments, two were ‘general palliative’ instruments (Patient Needs Assessment in Palliative care (PNAP) [18]; Sheffield Profile for Assessment and Referral for Care (SPARC) [19]) developed in mixed samples of people with advanced illnesses (including cancer). Eight were ‘advanced cancer’ instruments (Needs Assessment for Advanced Cancer Patients (NA-ACP) [17]; Needs Assessment for Advanced Lung Cancer Patients (NA-ALCP) [20]; Problems and Needs in Palliative Care Questionnaire (PNPC) [21]; Problems and Needs in Palliative Care Questionnaire–short version (PNPC-sv) [22]; Screen for Palliative and End-of-Life Care Needs in the Emergency Department (SPEED) [23]; Three Levels of Needs Questionnaire (3LNQ) [24]; name not given (Ndiok) [25]; Palliative Care Needs Assessment–English/Arabic Versions (PCNA-EAV) [26]). Fourteen were ‘all stage cancer’ instruments validated in a sample that included people with advanced cancer (Cancer Needs Distress Inventory (CaNDI) [27]; Comprehensive Needs Assessment Tool in Cancer (CNAT) [28, 29]; Psychosocial Needs Inventory (PNI) [30]; Electronic Holistic Needs Assessment (eHNA) [31]; 34-item Supportive Care Needs Survey (SCNS-SF34) [32]; Supportive Care Needs Assessment Tool for Indigenous People (SCNAT-IP) [33, 34]; Supportive Care Needs Survey–9-item Screening Tool (SCNS-ST9) [35]; 59-item Supportive Care Needs Survey (SCNS-LF59) [36]; Needs Evaluation Questionnaire (NEQ) [37,38,39]; Cancer Needs Questionnaire-Short Form (CNQ-sf )[40]; CancerSupportSource (CSS-25) [41, 42]; Bladder Cancer Needs Assessment Survey (BCNAS-32) [43]; Prostate Cancer Needs Questionnaire Version 2 (PCNQ V2) [44, 45]; You, Your family, and City of Hope are a team (YYFcore03) [46]). From these 14, 12 instruments were developed in a mixed sample of cancers, while the BCNAS-32 [43] and PCNQ V2 [45] were specifically developed for bladder and prostate cancer, respectively (Online Resource 6).
Eight instruments were developed in Australia [17, 20, 32, 33, 35, 36, 40, 44], five in the USA [23, 27, 41, 43, 46], three in the UK [19, 30, 31], two in Netherlands [21, 22], and one in each in Czech Republic [18], Denmark [24], Nigeria [25], Saudi Arabia [26], Republic of Korea [28], and Italy [37].
Instrument characteristics
Instrument length ranged from 9 to 138 items (Table 1). Seventeen were self-administered [17, 20,21,22, 24, 27, 30,31,32, 35,36,37, 40, 41, 43, 44, 46], and seven were interviewer-administered (e.g. by a clinician) [18, 19, 23, 25, 26, 28, 33]. For the 13 instruments that reported completion time, it ranged from 5 to 76 min [17,18,19, 22, 26, 27, 31,32,33, 36, 37, 41, 44]. Patients were asked to recall their needs during the following time periods: past 4 months (NA-ACP) [17], 1 month (n = 7 instruments) [19, 26, 28, 32, 33, 35, 36], few weeks (PNI) [30], 2 weeks (CaNDI) [27], 1 week (n = 2) [18, 24], and the present day (n = 2) [41, 44]. Ten instruments did not specify a recall period [20,21,22,23, 25, 31, 37, 40, 43, 46].
Fourteen instruments calculated subscale scores [17, 19,20,21, 24, 26, 27, 30, 32, 33, 36, 37, 40, 43], 12 scored each individual item [18, 19, 21,22,23, 25, 26, 31, 35, 37, 44, 46], and six calculated total scores [18, 27, 28, 31, 33, 41]. Eight instruments calculated more than one score type [18, 19, 21, 26, 27, 31, 33, 37]. Fourteen instruments asked patients to indicate – often through 4- or 5-point scales – the extent to which an item is a problem and/or the level of help needed [17, 18, 20, 23, 26, 28, 30,31,32, 35, 36, 40, 43, 44]. Four instruments used a dichotomous format to indicate (1) whether there is a problem and (2) whether they want help [21, 22, 25, 37]. Six instruments used a combination of these approaches, to indicate whether there is a problem, then how much help is needed, or vice versa [19, 24, 27, 33, 41, 46].
Eight instruments have been validated in languages other than English (Online Resource 7) [19, 22, 27, 28, 32, 35, 37, 40].
Instrument development
Table 2 details the item generation and reduction techniques used to develop each instrument. Online Resource 8 specifies the ‘other’ techniques. Online Resource 9 signposts to qualitative papers that informed an instrument’s development.
Item generation
Fifteen instruments used more than one item generation technique [17,18,19,20,21, 23,24,25,26,27,28, 30, 33, 43, 46] – the most common of which were amending items from an existing instrument (n = 11 instruments) [20, 22,23,24, 32, 33, 35, 36, 40, 44, 46]; using the literature (n = 10) [17,18,19, 24,25,26,27, 30, 43, 46]; and patient interviews and focus groups (n = 10) [17, 19, 21, 27, 28, 30, 33, 37, 41, 43]. Less common techniques included review of content of existing instruments (n=6) [18, 21, 23, 24, 26, 28]; consulting with an expert panel (n = 6) [19,20,21, 23, 26, 43]; health professional interviews and focus groups (n = 5) [17,18,19, 21, 27]; and informed by a conceptual model (n = 2) [25, 27].
Item reduction
Seventeen instruments reported an item reduction process [17,18,19,20,21,22, 27, 28, 32, 33, 35,36,37, 40, 41, 43, 44]. Eleven instruments reported more than one item reduction technique [18,19,20, 27, 28, 32, 33, 35,36,37, 41]. The most common techniques were factor analysis (n = 8 instruments) [17, 20, 32, 33, 35, 41, 43, 44]; item response frequencies (n = 6) [18, 20,21,22, 35, 40]; patient review (n = 5) [27, 28, 33, 36, 41]; expert review (n = 3) [18, 28, 36]; and test-retest reliability (n = 2) [20, 37].
Instrument content
The most frequently measured unmet need dimensions were psychological (n = 24 instruments) [17,18,19,20,21,22,23,24,25,26,27,28, 30,31,32,33, 35,36,37, 40, 41, 43, 44, 46]; healthcare (n = 22) [17,18,19,20,21, 23,24,25, 27, 28, 30,31,32,33, 35,36,37, 40, 41, 43, 44, 46]; activities of daily living (n = 21) [17,18,19,20,21,22,23,24,25,26,27,28, 30,31,32,33, 36, 37, 40, 41, 46]; and physical (n = 21) [17,18,19,20,21,22,23,24, 26,27,28, 30,31,32,33, 35,36,37, 40, 41, 46]. Further frequently measured dimensions comprised information (n = 19) [17,18,19,20,21,22, 25, 26, 28, 30, 32, 33, 35,36,37, 40, 43, 44, 46]; social (n = 18) [17,18,19,20,21,22,23,24,25, 27, 28, 30, 31, 37, 40, 41, 43, 44]; and sexual (n = 17) [19, 21, 22, 24,25,26,27, 30,31,32,33, 35, 36, 41, 43, 44, 46].
Of the included instruments, the PNPC [21] and PNI [30] were the most comprehensive, measuring all 11 unmet need dimensions, while CaNDI [27] measured 10. According to our criteria, SPEED [23] was the least comprehensive, measuring only five of the dimensions (Table 3, Online Resource 10).
Clinimetric properties
Methodological quality
SPARC [19] and SCNAT-IP [33] were rated adequate for PROM development. The remaining instruments were rated doubtful (n = 11) [17, 20, 21, 28, 32, 35,36,37, 40, 41, 44] or inadequate (n = 11) [18, 22,23,24,25,26,27, 30, 31, 43, 46], due to a lack of pilot testing; failing to ask patients about comprehensibility; or failure to ensure or clarify, methodological detail, such as involving two researchers in the analysis. SPARC [19] and CNAT [28] were rated adequate for content validity. The remaining were doubtful (n = 11) [17, 20,21,22, 27, 32, 35, 36, 40, 41, 44] or inadequate (n = 11) [18, 23,24,25,26, 30, 31, 33, 37, 43, 46], primarily due to an insufficient sample of patients and professionals being asked about item relevance and comprehensiveness.
Twelve instruments were rated very good (n = 6) [18, 28, 32, 37, 41, 46] or adequate (n = 6) [31, 33, 35, 36, 40, 44] for structural validity. The remaining were inadequate for failing to conduct a factor analysis, or conducting analysis with an insufficient sample size. Twenty instruments were rated very good for internal consistency [17, 18, 20,21,22,23, 26,27,28, 30,31,32,33, 36, 37, 40, 41, 43, 44, 46]. The remaining were doubtful (n = 2) [19, 25] or inadequate (n = 2) [24, 35] for not being clear about, or measuring, internal consistency. For reliability, CSS-25 [41] was rated very good, and six others, adequate [17, 18, 27, 37, 44, 46]. One instrument [26] was rated doubtful because systematic change had occurred between assessment time points, while the remaining were inadequate for failing to test patients at different time points.
PCNA-EAV [26] and NEQ [37] were rated adequate for measurement error. The remaining were rated inadequate for not calculating the standard error of measurement, smallest detectable change, limits of agreement, or the percentage of agreement. Seven instruments were rated very good for hypotheses testing [27, 28, 32, 33, 40, 41, 43]. One instrument [26] was doubtful due to insufficient information on the measurement properties of comparator instruments. The remaining instruments were rated inadequate for either no information on the measurement properties of comparator instruments or no assessment of known groups validity. None of the instruments were tested for responsiveness (Table 4, Online Resource 11).
GRADE levels of evidence
NA-ACP [17] and NA-ALCP [20] were the only instruments with high evidence for any clinimetric property, in both instances internal consistency (Table 5, Online Resource 12). CSS-25 [41] appeared strongest overall, with moderate evidence for four properties. CNAT [28] and SCNS-SF34 [32] had moderate evidence for three properties. Eight instruments had at least moderate evidence for two properties [17, 18, 27, 33, 37, 40, 43, 46]. Eight instruments had at least moderate evidence for only one property [20,21,22, 26, 30, 31, 36, 44], all being internal consistency. The remaining five instruments had low or very low evidence across all seven clinimetric properties [19, 23,24,25, 35]. With the exception of internal consistency (for which two and 17 instruments had high and moderate evidence, respectively), very low evidence was common across all clinimetric properties.
Quality of clinimetric properties
Across all properties, no instrument received an insufficient rating (Table 4, Online Resource 13). Five instruments had sufficient structural validity [18, 28, 37, 41, 46]. The remaining instruments were indeterminate, primarily due to a lack of confirmatory factor analysis (CFA) or use of item response theory (IRT). eHNA [31] and SCNS-SF34 [32] conducted IRT and CFA, respectively, but failed to report the information required for a sufficient rating. Nine instruments had sufficient internal consistency [17, 23, 28, 32, 33, 36, 41, 43, 44]. The remaining were indeterminate, due to not having at least low evidence for sufficient structural validity; not reporting Cronbach’s alpha; or Cronbach’s alpha ranged below and above 0.70 across all unidimensional scales. Five instruments had sufficient reliability [17, 27, 41, 44, 46]; as COSMIN does not specify that the intraclass correlation coefficients (ICC) need to be above 0.70 in each dimension, studies were rated sufficient if at least one ICC was >0.70. The remaining were rated indeterminate for not reporting ICC or weighted Kappa. All 24 instruments had indeterminate measurement error [17,18,19,20,21,22,23,24,25,26,27,28, 30,31,32,33, 35,36,37, 40, 41, 43, 44, 46] for failing to define a minimal important change. Thirteen instruments had sufficient hypotheses testing [18, 20,21,22, 26,27,28, 32, 33, 40, 41, 43, 44], as they reported results in accordance with their hypotheses, while the remaining were indeterminate for failing to define a hypothesis.
Feasibility
Twelve instruments reported varying levels of patient comprehensibility (Online Resource 14) [17, 19, 20, 24, 26, 27, 32, 33, 35,36,37, 44], while no instrument reported clinician comprehensibility. Only NA-ACP [17] and CaNDI [27] reported patients required mental and physical ability level, both considered by their authors to be understandable by > 90% of people aged 25–64 years.
Four instruments reported how to standardise scores, all using the same formula [32, 33, 35, 43]. Copyright was reported in five instruments [26, 31, 32, 35, 36]. Instrument access was available: within the paper (n = 11 instruments) [23, 25, 28, 30, 35,36,37, 40, 43, 44, 46]; as an appendix (n = 6) [20,21,22, 24, 26, 27]; to download online (n = 3) [19, 32, 41]; through another route (n = 3) [17, 31, 33]; and for PNAP [18] access was not reported.
Ten instruments were free to access [19, 31,32,33, 35,36,37, 40, 41, 44], while the remaining did not report cost of access. Five instruments were available in more than one format [27, 32, 33, 35, 36]. All except PCNA-EAV [26] (not reported) were stated to be available for use in a clinical setting. Sixteen instruments were also stated to be suitable for use in a research setting [17,18,19,20,21,22, 24, 25, 27, 28, 32, 35,36,37, 40, 41]. CSS-25 [41] reported additional usability in a community setting. No instrument reported requiring regulatory agency approval for use.
Interpretability
Through frequencies, mean and standard deviation, median, or range, 16 instruments reported the distribution of scores in the study population (Online Resource 15) [21, 22, 24,25,26,27,28, 30,31,32,33, 35, 37, 40, 41, 43]. Nine instruments reported a percentage of missing items, either through the percentage missing for each individual item or percentage of the sample that missed ≥ 1 item [19, 20, 26, 27, 33, 37, 41, 43, 44]. Floor and ceiling effects were not applicable to the four instruments that included only dichotomous response options [21, 22, 25, 37]. Four instruments reported that such effects were either not observed or had been addressed through item reduction [19, 27, 32, 33]. CNAT [28] reported considerable floor effect and little ceiling effect. Eight instruments presented scores available for relevant subgroups, such as gender, age, treatment, and cancer type [26,27,28, 30,31,32, 37, 40]. No instrument reported a minimal important change/difference or provided information on response shift.
Discussion
Summary of main findings
This systematic review aimed to identify available unmet need instruments targeted at, or applicable to, people with advanced cancer. Overall, we identified 24 instruments. These were predominantly developed for all stages of cancer, with only eight specifically focused on advanced cancer [17, 20,21,22,23,24,25,26]. Hence, most authors did not specifically report how these instruments perform in advanced cancer populations. This study extends the work of Tian et al. who evaluated the psychometric properties of needs assessment tools in cancer [12]. Though our focus on advanced cancer was more specific than the work of Tian et al., we still included 11 additional instruments [18, 22, 25, 26, 30, 31, 36, 41, 43, 44, 46].
Instrument development
Ten instruments incorporated patient discussions in their development, indicating consideration of the target population’s perspective. Of these, four also included health professional discussions or an expert panel to acquire both perspectives. However, according to the COSMIN criteria, the methodological quality of the included instruments was generally poor; indeed, SPARC [19], NA-ACP [17], and NA-ALCP [20] were the only instruments without very low evidence for both PROM development and content validity. Particularly, following development work, it was often unclear what constituted the final version of the instrument. This made aspects of our appraisal of the instruments challenging and would potentially have implications for others who might wish to use these instruments in research or practice.
Instrument content
For consistency, we mapped the questions in the instruments to pre-defined dimensions (rather than rely on authors’ self-reported dimensions). There was substantial heterogeneity in the number of dimensions of unmet need assessed by each instrument. Twenty instruments assessed ≥ 7 dimensions, with PNPC [21] and PNI [30] assessing all 11 dimensions, while SPEED [23] (5 dimensions), SCNS-ST9 [32], BCNAS-32 [43], and PCNQ V2 [44] (6 dimensions) assessed the lowest number of dimensions. Hence, some instruments do not offer patients the opportunity to indicate difficulties, or a desire for help with, at least five dimensions of unmet need, potentially providing an incomplete picture of unmet needs at the individual or population level.
Instrument quality
Despite the number of available instruments, methodological quality was variable. Only CSS-25 [41] (four properties), CNAT [28], and SCNS-SF34 [32] (three properties) had at least moderate evidence for three or more clinimetric properties. Though these three appear more clinimetrically robust than other instruments, they were developed for the whole cancer trajectory (rather than advanced cancer patients). Thus, it is unclear whether they are robust specifically for assessing unmet needs in advanced cancer. Of note, Moghaddam et al. argued, in the context of a systematic review of unmet needs in those with advanced cancer, that SCNS-SF34 neglects some dimensions of unmet need [8]. Since our search was completed in January 2021, the development of CancerSupportSource-15+ (CSS-15+), a shortened version of CSS-25, has been published [47]. The authors of that paper state that CSS-15+ is a brief, valid, and reliable multidimensional instrument that has strong correlation with CSS-25. As CSS-25 [41] was identified, clinimetrically, as the strongest instrument in the present review, CSS-15+ may warrant closer consideration.
Selecting an instrument for use
Our particular interest was to identify instruments which may be used to assess unmet needs in patients with advanced cancer. Recommendations for which instrument to use may be informed by robust development, comprehensive content, or strong methodological quality, as outlined above. However, no particular instrument stands out in all of these aspects. Thus, recommendations may also be informed by instrument burden and ease of administration. Instrument length, completion time, and availability in different formats are arguably particularly important considerations for advanced cancer patients, who may, for example, have a significant symptom burden. Accordingly, eHNA [31], SCNS-ST9 [35], and NEQ [37] have favourable characteristics, though are variable in their content and quality.
A clinical setting may have the capacity or desire to deliver services and supports to address patient needs. All instruments in our review can be used in a clinical setting, so when used, have the potential to inform the development of a future care plan. However, many instruments can also be used in a research setting, and some care may be needed here. A needs assessment may raise the awareness or expectations of the patient. As Ahmed et al. have noted, if help is not going to be offered once a need has been identified, it could be counterproductive [48].
With the growth in availability and efficacy of the new precision and biological therapies [4] for those with advanced cancer, it is important to understand how – and if – instruments perform in capturing the needs of those treated by these new treatments. However, as none of the instruments states development or validation in a such population, future research should explore this, especially given the unique adverse effect profiles of these treatments.
Reporting instrument development and validation
When using COSMIN to assess clinimetrics, it should be noted that we are not judging that something has not been done in a study; rather, we have made a judgement on what has been reported. Many development studies were published before the first iterations of the COSMIN checklist, and this may account, in part, for the low assessed quality. What has been reported for an instrument may be driven by what is deemed important by the authors and/or the word limit afforded in a medical journal. This may be insufficient to report the detail necessary for full COSMIN appraisal. In particular, methodological detail of development stages was often reported only very briefly. We would suggest that, in future, when reporting instrument development and validation, authors make use of supplementary material to provide additional methodological detail.
In 2019, a COSMIN study design checklist was established [49]. This allows authors to clarify the necessary detail for each stage of instrument development and validation. It would be helpful for scientific journals to require relevant studies be reported according to this checklist or that authors complete this checklist at submission and make it available.
Strengths and limitations
The present review benefitted from an extensive search, including consideration of palliative care literature. This allowed us to identify instruments appropriate to those in palliative care (i.e. PNAP and SPARC). Still, this review is not without limitations. Though thorough in our review process by searching several databases and handsearching reference lists and citations, we only included papers and instruments available in English. Thus, the possibility cannot be excluded that we have missed a relevant study or instrument not published in English. While we signpost to available language validations, we only assessed the clinimetric properties of the original English versions of each instrument. If assessed, these may have influenced the GRADE summary of evidence. Although authors were contacted, complete development work for eHNA and CNQ-sf was not available. Therefore, it was not possible to accurately assess certain clinimetric properties.
One of the challenges in this review was what precisely constitutes an unmet need. We took the view that this is something that a patient experiences as a problem and which they would like help or support with. This meant we excluded instruments that simply measured problems or symptoms and did not allow patients to infer a need for help. One could argue that having a significant problem equates to an implicit need, and the authors for some of the excluded instruments may consider them measures of unmet needs or needs assessment tools. Indeed, there are examples in the literature where scores above a specified cut-off on functioning or symptom scales within a validated instrument are taken to infer an unmet need [50]. This highlights a lack of clarity for when a need is considered to be unmet and how such an unmet need is identified or measured.
Equally, there are challenges around defining ‘advanced’ cancer. For this work, advanced cancer was operationalised in terms of disease stage (stage IV) and ‘status’ (metastatic), also acknowledging any cancers that were considered incurable or people who were undergoing palliative care. It is possible to define advanced cancer in other ways, such as likely prognosis, chance of disease eradication, or patient remaining life expectancy. Study populations tend not to be described in these terms, and these concepts are, arguably, harder to quantify or categorise than stage or metastatic status. Both ‘unmet need’ and ‘advanced cancer’ are key concepts in survivorship. Consensus definitions of these would be valuable.
Conclusion
We identified 24 instruments to measure unmet needs in people with advanced cancer. There is extensive heterogeneity in their development, content, and methodological quality. Moreover, the majority were not developed, or validated, with specific consideration of advanced cancer. The evolving management of advanced cancer, including the explosion in availability of precision and biological therapies, means it is important to consider whether existing instruments adequately capture the unmet needs of this population.
Data Availability
Not applicable
References
Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust TÅ, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019;20(11):1493–505. https://doi.org/10.1016/S1470-2045(19)30456-5.
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75. https://doi.org/10.1016/S0140-6736(17)33326-3.
Todd A, Groundwater PW, Gill JH. Anticancer therapeutics: from drug discovery to clinical applications. Hoboken: John Wiley & Sons; 2018.
Jackson SE, Chester JD. Personalised cancer medicine. Int J Cancer. 2015;137(2):262–6. https://doi.org/10.1002/ijc.28940.
Electronic medicines compendium. https://www.medicines.org.uk/emc/product/3856/smpc#UNDESIRABLE_EFFECTS. Accessed 18th February 2021
Electronic medicines compendium https://www.medicines.org.uk/emc/product/3885#UNDESIRABLE_EFFECTS. Accessed 18th February 2021
Pizzoli SF, Renzi C, Arnaboldi P, Russell-Edu W, Pravettoni G. From life-threatening to chronic disease: is this the case of cancers? A systematic review. Cogent Psychol. 2019;6(1):1577593. https://doi.org/10.1080/23311908.2019.1577593.
Moghaddam N, Coxon H, Nabarro S, Hardy B, Cox K. Unmet care needs in people living with advanced cancer: a systematic review. Support Care Cancer. 2016;24(8):3609–22. https://doi.org/10.1007/s00520-016-3221-3.
Wang T, Molassiotis A, Chung BP, Tan JY. Unmet care needs of advanced cancer patients and their informal caregivers: a systematic review. BMC Palliat Care. 2018;17(1):1–29. https://doi.org/10.1186/s12904-018-0346-9.
Mohammed S, Swami N, Pope A, Rodin G, Hannon B, Nissim R, et al. “I didn’t want to be in charge and yet I was”: bereaved caregivers' accounts of providing home care for family members with advanced cancer. Psychooncology. 2018;27(4):1229–36. https://doi.org/10.1002/pon.4657.
Yadav S, Heller IW, Schaefer N, Salloum RG, Kittelson SM, Wilkie DJ, et al. The health care cost of palliative care for cancer patients: a systematic review. Support Care Cancer. 2020;28:4561–73. https://doi.org/10.1007/s00520-020-05512-y.
Tian L, Cao X, Feng X. Evaluation of psychometric properties of needs assessment tools in cancer patients: a systematic literature review. PLoS One. 2019;14(1):e0210242. https://doi.org/10.1371/journal.pone.0210242.
Okediji PT, Salako O, Fatiregun OO. Pattern and predictors of unmet supportive care needs in cancer patients. Cureus. 2017;9(5):e1234. https://doi.org/10.7759/cureus.1234.
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Mokkink LB, De Vet HC, Prinsen CA, Patrick DL, Alonso J, Bouter LM, et al. COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1171–9. https://doi.org/10.1007/s11136-017-1765-4.
Prinsen CA, Mokkink LB, Bouter LM, Alonso J, Patrick DL, De Vet HC, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27(5):1147–57. https://doi.org/10.1007/s11136-018-1798-3.
Rainbird KJ, Perkins JJ, Sanson-Fisher RW. The needs assessment for advanced cancer patients (NA-ACP): a measure of the perceived needs of patients with advanced, incurable cancer. A study of validity, reliability and acceptability. Psychooncology. 2005;14(4):297–306. https://doi.org/10.1002/pon.845.
Buzgova R, Kozakova R, Sikorova L, Zelenikova R, Jarosova D. Development and psychometric evaluation of patient needs assessment in palliative care (PNAP) instrument. Palliat Support Care. 2016;14(2):129–37. https://doi.org/10.1017/s1478951515000061.
Ahmedzai SH, Payne S, Bestall JC, Ahmed N, Dobson K, Clark D, Noble B. Improving access to specialist palliative care: developing a screening measure to assess the distress caused by advanced illness that may require referral to specialist palliative care. 2005
Schofield P, Gough K, Ugalde A, Dolling L, Aranda S, Sanson-Fisher R. Validation of the needs assessment for advanced lung cancer patients (NA-ALCP). Psychooncology. 2012;21(4):451–5. https://doi.org/10.1002/pon.1902.
Osse BH, Vernooij MJ, Schadé E, Grol RP. Towards a new clinical tool for needs assessment in the palliative care of cancer patients: the PNPC instrument. J Pain Symptom Manag. 2004;28(4):329–41. https://doi.org/10.1016/j.jpainsymman.2004.01.010.
Osse BH, Vernooij-Dassen MJ, Schadé E, Grol RP. A practical instrument to explore patients’ needs in palliative care: the problems and needs in palliative care questionnaire—short version. Palliat Med. 2007;21(5):391–9. https://doi.org/10.1177/02F0269216307078300.
Richards CT, Gisondi MA, Chang CH, Courtney DM, Engel KG, Emanuel L, et al. Palliative care symptom assessment for patients with cancer in the emergency department: validation of the screen for palliative and end-of-life care needs in the emergency department instrument. J Palliat Med. 2011;14(6):757–64. https://doi.org/10.1089/jpm.2010.0456.
Johnsen AT, Petersen MA, Pedersen L, Groenvold M. Development and initial validation of the three-levels-of-needs questionnaire for self-assessment of palliative needs in patients with cancer. J Pain Symptom Manag. 2011;41(6):1025–39. https://doi.org/10.1016/j.jpainsymman.2010.08.013.
Ndiok A, Ncama B. Assessment of palliative care needs of patients/families living with cancer in a developing country. Scand J Caring Sci. 2018;32(3):1215–26. https://doi.org/10.1111/scs.12568.
Volker SE. Development and validation of a palliative care needs assessment instrument-English/Arabic versions (PCNA-EAV), for use with patients with advanced cancer: The University of Alabama at Birmingham; 2010.
Lowery AE, Greenberg MA, Foster SL, Clark K, Casden DR, Loscalzo M, et al. Validation of a needs-based biopsychosocial distress instrument for cancer patients. Psychooncology. 2012;21(10):1099–106. https://doi.org/10.1002/pon.2008.
Shim EJ, Park JH, Park JH. Comprehensive needs assessment tool in cancer (CNAT): the development and validation. Support Care Cancer. 2011;19(12):1957–68. https://doi.org/10.1007/s00520-010-1037-0.
Yang GM, Pang GS, Lee GL, Neo PS, Wong YY, Qu DL, et al. Validation of the comprehensive needs assessment tool in patients with advanced cancer. Indian J Palliat Care. 2019;25(3):374–8. https://doi.org/10.4103/ijpc.ijpc_38_19.
McIllmurray MB, Thomas C, Francis B, Morris S, Soothill K, Al-Hamad A. The psychosocial needs of cancer patients: findings from an observational study. Eur J Cancer Care. 2001;10(4):261–9. https://doi.org/10.1046/j.1365-2354.2001.00280.x.
Snowden A, Fleming M. Validation of the electronic holistic needs assessment. Springerplus. 2015;4(1):1. https://doi.org/10.1186/s40064-015-1401-0.
Boyes A, Girgis A, Lecathelinais C. Brief assessment of adult cancer patients’ perceived needs: development and validation of the 34-item supportive care needs survey (SCNS-SF34). J Eval Clin Pract. 2009;15(4):602–6. https://doi.org/10.1111/j.1365-2753.2008.01057.x.
Garvey G, Beesley VL, Janda M, Jacka C, Green AC, O’Rourke P, et al. The development of a supportive care needs assessment tool for Indigenous people with cancer. BMC Cancer. 2012;12(1):1. https://doi.org/10.1186/1471-2407-12-300.
Garvey G, Beesley VL, Janda M, O'Rourke PK, He VY, Hawkes AL, et al. Psychometric properties of an Australian supportive care needs assessment tool for Indigenous patients with cancer. Cancer. 2015;121(17):3018–26. https://doi.org/10.1002/cncr.29433.
Girgis A, Stojanovski E, Boyes A, King M, Lecathelinais C. The next generation of the supportive care needs survey: a brief screening tool for administration in the clinical oncology setting. Psychooncology. 2012;21(8):827–35. https://doi.org/10.1002/pon.1973.
Bonevski B, Sanson-Fisher R, Girgis A, Burton L, Cook P, Boyes A. Supportive care review group. Evaluation of an instrument to assess the needs of patients with cancer. Cancer. 2000;88(1):217–25. https://doi.org/10.1002/(SICI)1097-0142(20000101)88:1%3C217::AID-CNCR29%3E3.0.CO;2-Y.
Tamburini M, Gangeri L, Brunelli C, Beltrami E, Boeri P, Borreani C, et al. Assessment of hospitalised cancer patients' needs by the needs evaluation questionnaire. Ann Oncol. 2000;11(1):31–8. https://doi.org/10.1023/A:1008396930832.
Annunziata MA, Muzzatti B, Altoè G. A contribution to the validation of the needs evaluation questionnaire (NEQ): a study in the Italian context. Psychooncology. 2009;18(5):549–53. https://doi.org/10.1002/pon.1445.
Bonacchi A, Miccinesi G, Galli S, Primi C, Chiesi F, Lippi D, et al. use of the needs evaluation questionnaire with cancer outpatients. Support Care Cancer. 2016;24(8):3507–15. https://doi.org/10.1007/s00520-016-3176-4.
Cossich T, Schofield P, McLachlan SA. Validation of the cancer needs questionnaire (CNQ) short-form version in an ambulatory cancer setting. Qual Life Res. 2004;13(7):1225–33. https://doi.org/10.1023/B:QURE.0000037496.94640.d9.
Miller MF, Mullins CD, Onukwugha E, Golant M, Buzaglo JS. Discriminatory power of a 25-item distress screening tool: a cross-sectional survey of 251 cancer survivors. Qual Life Res. 2014;23(10):2855–63. https://doi.org/10.1007/s11136-014-0742-4.
Buzaglo JS, Zaleta AK, McManus S, Golant M, Miller MF. CancerSupportSource®: validation of a revised multi-dimensional distress screening program for cancer patients and survivors. Support Care Cancer. 2020;28(1):55–64. https://doi.org/10.1007/s00520-019-04753-w.
Leung TM, Benn E, Galsky M, Latini DM, Goltz HH, Lee CT, et al. Examining the psychometric properties of the bladder cancer needs assessment survey. JOJ Urol Nephrol. 2017;4:555627.
Duke JM, Treloar CJ, Byles JE. Evaluation of a revised instrument to assess the needs of men diagnosed with prostate cancer. Support Care Cancer. 2005;13(11):895–903. https://doi.org/10.1007/s00520-005-0808-5.
Duke JM, Treloar CJ, Byles JE. Evaluation of an instrument to assess the needs of men diagnosed with prostate carcinoma: an assessment of the validity and reliability of a self-administered questionnaire developed to measure the needs experienced by men diagnosed with prostate carcinoma. Cancer. 2003;97(4):993–1001. https://doi.org/10.1002/cncr.11156.
Wong FL, Stiller T, Obenchain R, David C, Patel SK, Xie B, et al. Validation of a biopsychosocial distress screening tool,“you, your family and coh are a team”. Psychooncology. 2019;28(12):2396–405. https://doi.org/10.1002/pon.5266.
Zaleta AK, McManus S, Fortune EE, DeRosa BW, Buzaglo JS, Olson JS, et al. CancerSupportSource®-15+: development and evaluation of a short form of a distress screening program for cancer patients and survivors. Support Care Cancer. 2021;14:1–9. https://doi.org/10.1007/s00520-021-05988-2.
Ahmed N, Hughes P, Winslow M, Bath PA, Collins K, Noble B. A pilot randomized controlled trial of a holistic needs assessment questionnaire in a supportive and palliative care service. J Pain Symptom Manag. 2015;50(5):587–98. https://doi.org/10.1016/j.jpainsymman.2015.05.010.
Mokkink LB, Prinsen CA, Patrick DL, Alonso J, Bouter LM, de Vet HC, Terwee CB. COSMIN study design checklist for patient-reported outcome measurement instruments. 2019
Jansen F, Snyder CF, Leemans CR. Verdonck–de Leeuw IM. Identifying cutoff scores for the EORTC QLQ-C30 and the head and neck cancer–specific module EORTC QLQ-H&N35 representing unmet supportive care needs in patients with head and neck cancer. Head Neck. 2016 Apr;38(S1):E1493–500. https://doi.org/10.1002/hed.24266.
Acknowledgements
We would like to thank Newcastle University Senior Library Assistant, Bogdan Metes, for his assistance with the development of the search strategy.
Code availability
Not applicable
Funding
This project was supported by funding from the Macmillan Cancer Support (FO-7165351) and the Brain Tumour Charity (GN-000435).
Author information
Authors and Affiliations
Contributions
All authors were involved in the conceptualisation of the review. BR conducted the searches, while BR and LC screened the results. BR, AT, and LS conducted the data extraction, analysis, and interpretation. BR drafted the manuscript, while LC contributed to the introduction. All authors reviewed, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rimmer, B., Crowe, L., Todd, A. et al. Assessing unmet needs in advanced cancer patients: a systematic review of the development, content, and quality of available instruments. J Cancer Surviv 16, 960–975 (2022). https://doi.org/10.1007/s11764-021-01088-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11764-021-01088-6