Abstract
Objective
There are no reports from Japan showing the effects of using the thromboelastography algorithm on transfusion requirements after Intensive Care Unit (ICU) admission, and post-implementation knowledge regarding the thromboelastography algorithm under the Japanese healthcare system is insufficient. Therefore, this study aimed to clarify the effect of the TEG6s thromboelastography algorithm on transfusion requirements for patients in the ICU after cardiac surgery.
Methods
We retrospectively compared the requirements for blood transfusion up to 24 h after ICU admission using the thromboelastography algorithm (January 2021 to April 2022) (thromboelastography group; n = 201) and specialist consultation with surgeons and anesthesiologists (January 2018 to December 2020) (non-thromboelastography group; n = 494).
Results
There were no significant between-group differences in terms of age, height, weight, body mass index, operative procedure, duration of surgery or cardiopulmonary bypass, body temperature, or urine volume during surgical intervention. Moreover, there was no significant between-group difference in the amount of drainage at 24 h after ICU admission. However, crystalloid and urine volumes were significantly higher in the thromboelastography group than in the non-thromboelastography group. Additionally, fresh-frozen plasma (FFP) transfusion volumes were significantly lower in the thromboelastography group. However, there were no significant between-group differences in red blood cell count or platelet transfusion volume. After variable adjustment, the amount of FFP used from the operating room to 24 h after ICU admission was significantly reduced in the thromboelastography group.
Conclusions
The thromboelastography algorithm optimized transfusion requirements at 24 h after admission to the ICU following cardiac surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Impaired hemostasis can contribute to intra- and postoperative bleeding and is associated with increased morbidity and mortality [1]. In general, standard laboratory tests include the activated partial thromboplastin time, prothrombin time, international normalized ratio, platelet count, and plasma fibrinogen. However, none of these tests were developed to predict bleeding or guide coagulation management in the surgical setting [2], and the usefulness of general coagulation tests in the diagnosis and treatment of perioperative coagulopathy remains unclear [3,4,5,6]. In contrast, coagulation monitoring through viscoelastic techniques, such as thromboelastography (TEG, TEG6s; Hemonetics LLC, Tokyo, Japan), has evolved into a point-of-care method that can detect the contribution of cellular and plasma components to hemostasis and is useful in reducing blood transfusion volumes [7]. TEG overcomes some of the limitations associated with conventional laboratory tests, shortens the time to return test results, assists the diagnosis of coagulopathy, and provides a coagulation function that integrates small changes in coagulopathy and platelet function.
In recent years, this transfusion algorithm using TEG has been shown to be superior to empirical hemostatic therapy based on clinical judgment. There is increasing recognition that the early diagnosis of hemostatic pathology and effective transfusion therapy is of clinical importance [8,9,10]. However, even after the introduction of TEG6s in Japan in 2015, blood transfusion judgment based on viscoelastic testing is not sufficiently widespread in the intensive care units (ICU). This is primarily because empiric transfusion is often strongly reflected in daily clinical care (e.g., blood transfusion based on the rule of thumb and determination of clotting ability based on central laboratory data alone).
No reports from Japan have shown the effects of using the TEG6s algorithm on transfusion requirements in the operating room and after ICU admission, and knowledge regarding the post-implementation evaluation of the TEG6s algorithm under the Japanese healthcare system is insufficient. To assess this, we designed a retrospective study that compared transfusion requirements during the first 24 h after ICU admission between patients who received blood transfusions using the TEG algorithm and those who received blood transfusions after consultation between cardiac surgeons and anesthesiologists.
Methods
Ethics statements
This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. This study also conformed to the standards of the Declaration of Helsinki and was approved by the ethics committee of Nagoya University Hospital (approval number: 2022–0246). Individual informed consent was obtained from the patients using an opt-out method of enrollment via the hospital’s website.
Study design and population
The primary endpoint of this single-center retrospective cohort study was transfusion requirements up to 24 h after ICU admission, assessed using the inverse probability weighting (IPW) method. From the sample of patients who underwent cardiac surgery, those who were introduced to the TEG6s protocol upon admission to the ICU (TEG group) were compared to those transfused after consultation between cardiac surgeons and anesthesiologists (non-TEG group). In January 2021, we began using the TEG6s algorithm for blood transfusion after ICU admission of patients who underwent cardiopulmonary bypass (CPB). Therefore, patients assessed from January 2021 to April 2022 were included in the TEG group, and those assessed from January 2018 to December 2020 were included in the non-TEG group.
TEG6s protocol
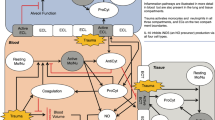
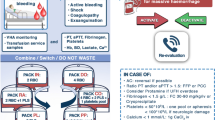
The TEG protocol is shown in Fig. 1. Red blood cell (RBC) transfusions are hemoglobin-triggered, and the TEG results are unaffected. For the values included in the algorithm, we evaluated several studies and adopted values that are commonly used and easy for cardiac surgeons and intensivists to understand [11,12,13,14]. TEG6s displays four waveforms: Citrated Kaolin (CK), Citrated Kaolin with Heparinase (CKH), Citrated Rapid TEG (CRT), and Citrated Functional Fibrinogen (CFF). By checking the Reaction time (R) and Maximum amplitude (MA) of each waveform, it is possible to determine whether FFP, PC, and fibrinogen preparation is needed, and whether heparin remains. R is the reaction time, expressed in minutes, and is the parameter that most strongly reflects the coagulation factor from the start of measurement to the rise in viscosity. This is the time of thrombus formation when thrombin is produced, and fibrin is formed. R > 10 min indicates a deficiency of coagulation factors and requires the use of FFP [12, 13]. In contrast, MA indicates clot strength and accounts for 80% platelets and 20% fibrin, expressed in mm. CFF-MA is used to ascertain clotting strength with fibrinogen alone, while other MAs are used to ascertain clotting strength by platelets and fibrinogen to determine the need for fibrinogen and platelet transfusion. CFF-MA < 12 indicates that fibrinogen replacement is required [11]. CRT-MA < 48 indicates insufficient clot strength by platelets and, thus, indicates the need for platelet transfusion [13].
TEG6s protocol in the intensive care unit. PB, cardiopulmonary bypass; CBC, complete blood count including red blood cell and platelet counts; PM, Platelet Mapping by TEG6s [15]; FibCare, a device that can rapidly measure the fibrinogen levels in the operating room [10]; ACT, activated coagulation time; R, reaction time of TEG6s; MA, maximum amplitude of TEG6s; FFP, fresh-frozen plasma; PC, platelet concentrate; Cryo, cryoprecipitate. The following were the TEG6s measurement items: CK-R, CKH-R, CRT-MA, and CFF-MA. For the values included in the algorithm, we consulted the academic literature and adopted commonly used values [11,12,13,14]
Exclusion criteria
The following operative methods, in which large amounts of intraoperative and postoperative blood transfusions were predicted preoperatively, were excluded: thoracoabdominal aortic replacement with or without abdominal branch reconstruction and patients with bleeding diathesis, heart transplantation, and revision of a left ventricular assist device, such as from the Heart-Mate II to Heart-Mate III (Abbott Laboratories, Chicago, IL, USA).
Data extraction
The following information was obtained from the anesthesia recording system database (ORSYS and ACSYS; Philips Healthcare, Best, Netherlands) and electronic medical record system (HOPE/EGMAIN-GX; Fujitsu, Tokyo, Japan): age; height; weight; sex; operative method; operative time; anesthetic time; CPB time; minimum body temperature at CPB (skin, bladder, and nasopharynx); total volumes of RBCs, fresh-frozen plasma (FFP), and platelet concentrate (PC) transfused in the operating room; total volumes of RBCs, FFP, and PC transfused in the first 24 h after ICU admission; volume of blood lost in the operating room after weaning from CPB, total volume of intraoperative fluid, total volume of intraoperative urine, and blood cells in the first 24 h after admission to the ICU; total volumes of FFP and PC transfusions; total drain volume up to 24 h after admission to the ICU; crystalloid volume up to 24 h after admission to the ICU; urine volume up to 24 h after admission to the ICU; ICU length of stay; days from surgery to discharge; and 30-day mortality.
General anesthesia
The basic type of anesthesia did not change during the study period. Standard monitoring was performed in all patients undergoing cardiovascular surgery. Fentanyl and Midazolam were administered intravenously to induce general anesthesia. Remifentanil and Rocuronium were additionally used to facilitate tracheal intubation, and general anesthesia was maintained with air, oxygen, Remifentanil, and volatile anesthetics. Porcine heparin (300 U/kg) was administered before starting cannulation for CPB, and additional heparin boluses (50 U/kg) were administered to maintain an activated coagulation time > 480 s. Protamine (3 mg/kg) was administered to antagonize the heparin effect. RBCs were transfused to maintain a hemoglobin level > 7–8 mg/dL during CPB. Blood sampling and platelet mapping or FibCare (Atom Medical, Tokyo, Japan) were used to measure the fibrinogen levels during cardiopulmonary withdrawal [11, 15]. For the TEG group, we used a TEG algorithm similar to that used in the ICU. CPB was terminated with inotropic drug support, and patients were admitted to the ICU under deep sedation without extubation.
Statistical analysis
Qualitative variables and demographic data, such as age, weight, height, and body mass index (BMI), were expressed as means (standard deviations) or medians [interquartile ranges] and compared between the groups using the t test or Wilcoxon rank-sum test, based on the distribution of each variable. Categorical data were expressed as numbers and percentages (%), and were analyzed using the Pearson Chi-square test. To examine changes in the blood transfusion volume before and after the introduction of the TEG6s algorithm, multivariable regression analysis was performed, with the blood transfusion volume as the dependent variable, and “before and after the introduction of the algorithm” as the independent variable, adjusting for age, sex, BMI, the type of surgical procedure, surgical duration, CPB time, blood loss, and urine volume. Owing to the positive skewness of the data distribution, we log-transformed the operative time, CPB time, and blood loss. Multivariable regression analyses were performed when the linearity assumption was followed, the residuals were normally distributed, and there were no strongly leveraged outliers. Three cases were excluded from the multivariable regression models due to the influence of the outliers. In addition, a logistic regression analysis was used to calculate the propensity scores, after which the IPW method was employed. Variables used to calculate the propensity score included age, sex, BMI, type of surgical procedure, surgical duration, CPB time, blood loss, and urine volume.
A p-value < 0.05 was deemed statistically significant. All statistical analyses were performed using Stata 17 MP (StataCorp, College Station, TX, USA).
Results
Between January 1, 2018, and April 30, 2022, 754 patients were evaluated, and 59 patients were excluded based on the exclusion criteria. The TEG and non-TEG groups included 201 and 494 patients, respectively. Finally, 695 patients were included in the analysis (Fig. 2). The patient characteristics are presented in Table 1. There were no significant differences in age, height, weight, BMI, or operative procedure between the two groups.
Flow diagram. During the study period, 754 patients underwent cardiac surgery with cardiopulmonary bypass and 59 patients met the exclusion criteria; thus, 695 patients were included. In total, 494 and 201 were classified in the non-TEG and TEG groups, respectively. All classified cases were included in the analysis as there were no omissions due to insufficient data. TEG, thromboelastography
The analysis items after ICU admission are shown in Table 2 and Fig. 3. Although there was no significant difference in the 24-h drainage volume, the 24-h crystalloid fluid and urine volumes were significantly higher in the TEG than in the non-TEG group (Table 2). In contrast, RBC transfusion was significantly lower in the TEG than in the non-TEG group; however, there was no significant difference between FFP and PC use. Multivariate regression analysis was performed after adjusting for age, sex, the type of surgery, BMI, operation time, CPB time, blood loss, and urine volume. Multivariable analysis showed that the FFP transfusion volume was significantly lower in the TEG than in the non-TEG group (Table 2 and Fig. 3), and the time to extubation and ICU length of stay were significantly shorter (Table 2). There was no significant difference in 30-day or in-hospital mortality between the two groups; however, the time from surgery to discharge was significantly shorter in the TEG than in the non-TEG group. The results of the IPW method, which was the final analysis (Fig. 4), indicated that FFP in the ICU after the introduction of the TEG protocol showed a significant reduction in usage. The distribution of the propensity score is presented in Online Resource 1-a and 1-b, and standardized differences between the covariates before and after the algorithm implementation are presented in Online Resource 2. The covariates were well balanced after IPW.
Forest plot according to multivariable regression analysis. To examine changes in the blood transfusion volume before and after the introduction of the TEG6s algorithm, multivariable regression analysis was performed, with blood transfusion volume as the dependent variable, and “before and after the introduction of the algorithm” as the independent variable, adjusting for age, sex, body mass index, operative time, ventilation time, and blood loss. “Before” referred to the non-TEG group, whereas “after” referred to the TEG group. TEG, thromboelastography; RCC, red cell concentrate; FFP, fresh-frozen plasma; PC, platelet concentrate
Forest plot according to propensity scores and the inverse probability weighting method. To examine changes in the blood transfusion volume before and after the introduction of the TEG6s algorithm, propensity scores and the inverse probability weighting method was performed, with blood transfusion volume as the dependent variable, and “before and after the introduction of the algorithm” as the independent variable, adjusting for age, sex, body mass index, operative time, ventilation time, and blood loss. “Before” referred to the non-TEG group, whereas “after” referred to the TEG group. TEG, thromboelastography; RCC, red cell concentrate; FFP, fresh-frozen plasma; PC, platelet concentrate
Operation-related items, including blood transfusions in the operating room, are presented in Table 3 and Fig. 3. There were significant differences in the duration of stay in the operating room and duration of anesthesia between the two groups; however, there were no significant differences in the operative time, CPB time, body temperature, or urine volume during the surgical intervention. The TEG group used significantly fewer RBCs and less FFP than the non-TEG group, and there were no significant differences in the PC or cryoprecipitate between the two groups. Multivariate regression analysis performed after adjusting for the same factors as before showed that the TEG group had a significant decrease in RBC and FFP use compared to the non-TEG group (Fig. 3). The results of the IPW method, which is the final analysis (Fig. 4), indicated that RBC and FFP in the operating room after the introduction of the TEG protocol showed a significant reduction in usage. Balance checks for the propensity score distributions and covariates are presented in Online Resource 1a, 1b, and 2. In addition, transfusion use from the operating room up to 24 h after ICU admission was significantly reduced in both RBC and FFP in the TEG group than in the non-TEG group. Similar to the total transfusion use after adjusting for variables, the total volume of RBCs and FFP from the operating room to 24 h of ICU admission was significantly reduced in the TEG group.
Discussion
The novelty of this study lies in the finding that the TEG6s algorithm in the Japanese healthcare system contributed to optimizing the amount of blood transfusions needed in the ICU. In this study, there were no significant differences between the two groups in terms of surgical technique, CPB time, or the amount of ICU drainage 24 h after surgery; however, there was a significant reduction in the amount of FFP transfused from the first 24 h after ICU admission. The drainage volume did not differ significantly between the two groups, and the TEG group had a significantly lower FFP dose and a trend toward lowering the RBC counts, suggesting that the TEG6s algorithm is also effective in the ICU. According to Maeda et al., the amount of blood transfused in the ICU is higher than that at the time of surgery [16]. With the arrival of a super-aging society in Japan, the indications for cardiac surgery among elderly patients are expanding along with advances in surgical techniques and anesthesia, and the demand for blood transfusions is increasing, since cardiac surgery is the sector that requires the most blood transfusions [17]. Therefore, the rationalization of transfusion volume in the ICUs has an important role in patient blood management (PBM).
Weber et al. reported a decrease in perioperative RBC and FFP transfusions in a randomized controlled trial of 100 adults undergoing cardiac surgery (50 each in the algorithm and conventional transfusion groups) [8]. The present study differs from that of Weber et al. insofar as that RBC transfusions did not decrease. This may be attributed to differences in the chest drainage volume between the studies. Weber et al. also used the hemoglobin levels as an algorithm to perform blood transfusions, but the traditional arm of their study may have originally had higher drain volumes. Drainage at 6 h after ICU admission was greater than 100 mL/h in the non-TEG group, suggesting that even the non-TEG group, which tended to have a larger volume of blood transfusions, had a larger volume of drainage. Although our study had a retrospective design, both groups had a sufficient number of patients (over 200), the IPW method was used to ensure balance between the two groups, and both groups had approximately 500 mL of drainage at 24 h after ICU admission. Similar to previous studies [8], we found that operating room transfusion requirements were significantly reduced in the TEG group. The current study indicated that the use of TEG in the ICU can reduce FFP and, in terms of total volume, including the volume of blood transfused in the operating room, significantly reduce the volume of blood transfused, both RBC and FFP, compared to the non-TEG group. Notably, previous reports strongly support our findings [10, 18].
In Table 2, the number of patients who did not receive RBC in the non-TEG, TEG, and Total groups was 243, 116, and 359, respectively. The number of patients who did not receive FFP was 264, 115, and 379, respectively. The number of patients who did not receive PC was 427, 178, and 605, respectively. Further, the number of patients who did not receive RBC in the non-TEG, TEG, and Total groups was 210, 120, and 330, respectively (Table 3). Moreover, the number of patients who did not receive FFP was 154, 107, and 261, respectively. Finally, the number of patients who did not receive PC was 306, 121, and 427, respectively. Thus, the items, in which more than half of the patients did not receive transfusion, had a median of zero.
This study assessed patients admitted to an ICU managed by an intensive care physician both before and after the introduction of the viscoelastic transfusion algorithm. Using the TEG6s algorithm in blood transfusion selection after cardiac surgery can be developed into a common protocol that includes intensive care physicians as well as surgeons and nurses. Especially, even in the ICUs where there is no dedicated ICU intensivist or where surgeons are primarily in charge, excessive transfusions can be reduced, and appropriate transfusions can be administered. The TEG6s algorithm is also important in terms of its contribution to PBM from the perspective of patient quality and safety. Each transfusion guideline recommends the use of viscoelasticity in cardiac surgery [19, 20]. Meeting the common medical standard of using viscoelastic testing to optimize blood transfusions contributes to PBM and provides patients with high quality of medical care and safety, including transfusion-related complications.
Careful discussion is necessary, although significant results exist for the crystalloid fluid and urine volumes, and no significant results were observed in the usage of PC. As stated in the ICU-TEG protocol, if no problems exist with the results of TEG, a higher dose of crystalloid fluid would be used in cases of low blood pressure. The dosage of crystalloid fluid has increased since the FFP at early admission in the ICU has been replaced by crystalloid fluid. Increased urine output could also be attributed to this. As platelet transfusion is routinely ordered and administered in the case of major vessel surgery or re-thoracotomy in Japan, such routine practice is the consensus between surgeons and anesthesiologists at each hospital. There was a separate rule that excluded the platelet administration algorithm in aortic operations, such as arch replacement. Therefore, there was no significant difference in the platelet transfusion volume between the two groups. The algorithm in the ICU defines PC use, but there were no significant between-group differences. Although the time to extubation, ICU length of stay, and time from surgery to discharge were significantly shorter in the TEG than in the non-TEG group, attention should be paid to the interpretation of these results as there are various possible influencing factors, such as the ICU discharge date determined by negotiation with the general ward and discharge dates that are artificially adjusted according to the patient’s wishes as well as changes in hospital-wide bed management under coronavirus disease 2019 restrictions.
This study has several limitations. First, this study had a retrospective design. We considered it difficult to conduct a prospective study in a situation where the use of viscoelasticity testing in the operating room is currently recommended. Although this was a retrospective study, both groups included more than 200 patients, balance between the 2 groups was ensured by the IPW technique, and our results suggested a solid contribution to PBM, a finding that will help other institutions. Second, data on the medical background of patients was limited. Medical treatment and health insurance systems vary widely between the United States, Europe, and Asia. Therefore, the costs associated with TEG were not calculated in this study. Finally, the TEG algorithm used in this study was developed through consultation between cardiac surgeons and anesthesiologists at our hospital. The algorithm should be modified according to the thinking of each institutional department.
Conclusion
Viscoelastic testing algorithms optimize the need for blood transfusion up to 24 h after ICU admission. Consequently, the use of viscoelastic testing also contributes to PBM from the viewpoint of medical quality and patient safety, such as the provision of blood transfusion complications and appropriate medical quality to patients.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TEG:
-
Thromboelastography
- ICU:
-
Intensive care unit
- CPB:
-
Cardiopulmonary bypass
- PBM:
-
Patient blood management
- RBC:
-
Red blood cell
- FFP:
-
Fresh-frozen plasma
- PC:
-
Platelet concentrate
- BMI:
-
Body mass index
References
Hardy JF, de Moerloose P, Samama M. Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth. 2004;51:293–310.
Benes J, Zatloukal J, Kletecka J. Viscoelastic methods of blood clotting assessment - a multidisciplinary review. Front Med (Lausanne). 2015;2:62.
Levy JH, Szlam F, Wolberg AS, Winkler A. Clinical use of the activated partial thromboplastin time and prothrombin time for screening: a review of the literature and current guidelines for testing. Clin Lab Med. 2014;34:453–77.
Mann KG, Brummel K, Butenas S. What is all that thrombin for? J Thromb Haemost. 2003;1:1504–14.
DeLoughery TG. Coagulation defects in trauma patients: etiology, recognition, and therapy. Crit Care Clin. 2004;20:13–24.
Haas T, Fries D, Tanaka KA, Asmis L, Curry NS, Schöchl H. Usefulness of standard plasma coagulation tests in the management of perioperative coagulopathic bleeding: is there any evidence? Br J Anaesth. 2015;114:217–24.
Görlinger K, Dirkmann D, Hanke AA, Kamler M, Kottenberg E, Thielmann M, et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011;115:1179–91.
Weber CF, Görlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117:531–47.
Girdauskas E, Kempfert J, Kuntze T, Borger MA, Enders J, Fassl J, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg. 2010;140:1117–24.
Karkouti K, McCluskey SA, Callum J, Freedman J, Selby R, Timoumi T, et al. Evaluation of a novel transfusion algorithm employing point-of-care coagulation assays in cardiac surgery: a retrospective cohort study with interrupted time-series analysis. Anesthesiology. 2015;122:560–70.
Tamura T. Predicting results of fibrinogen and platelet levels by TEG6s during cardiopulmonary bypass: a pilot study. J Clin Anesth. 2019;58:59–60.
Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–9.
Royston D, von Kier S. Reduced haemostatic factor transfusion using heparinase-modified thrombelastography during cardiopulmonary bypass. Br J Anaesth. 2001;86:575–8.
Stensballe J, Ostrowski SR, Johansson PI. Viscoelastic guidance of resuscitation. Curr Opin Anaesthesiol. 2014;27:212–8.
Yamada A, Tamura T, Imaizumi T, Kubo Y, Nishiwaki K (2023) Fibcare® shows correlation with fibrinogen levels by the Clauss method during cardiopulmonary bypass. Nagoya J Med Sci. In press
Henderson RA, Mazzeffi MA, Strauss ER, Williams B, Wipfli C, Dawood M, et al. Impact of intraoperative high-volume autologous blood collection on allogeneic transfusion during and after cardiac surgery: a propensity score matched analysis. Transfusion. 2019;59:2023–9.
Maeda H, Masahiro A, Tanaka A, Makino S, Kino S. The 2013 Nationwide Survey on massive transfusion cases with 10 or more units of red blood cells in Japan. Jpn J Transfus Cell Ther. 2015;61:409–15.
Pearse BL, Smith I, Faulke D, Wall D, Fraser JF, Ryan EG, et al. Protocol guided bleeding management improves cardiac surgery patient outcomes. Vox Sang. 2015;109:267–79.
Kozek-Langenecker SA, Afshari A, Albaladejo P, Santullano CA, De Robertis E, Filipescu DC, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30:270–382.
American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice guidelines for perioperative blood management: an updated report by the American society of anesthesiologists task force on perioperative blood management*. Anesthesiology. 2015;122:241–75.
Acknowledgements
We thank all anesthesiology and cardiac surgery staff members of Nagoya University Hospital.
Funding
Support was provided solely by institutional and/or departmental sources, and this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
TT, SS, TF, TH, YS, YN, and MM provided medical care. IT and YK performed data analysis. TT wrote the first draft of the manuscript and KN made the critical revisions. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study conformed to the standards of the Declaration of Helsinki and was approved by the ethics committee of Nagoya University Hospital (approval number: 2022–0246). Individual informed consent was obtained from the patients using an opt-out method of enrollment via the hospital’s website.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tamura, T., Suzuki, S., Fujii, T. et al. Thromboelastographic evaluation after cardiac surgery optimizes transfusion requirements in the intensive care unit: a single-center retrospective cohort study using an inverse probability weighting method. Gen Thorac Cardiovasc Surg 72, 15–23 (2024). https://doi.org/10.1007/s11748-023-01941-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-023-01941-8