Abstract
Objectives
Doxorubicin is a type of effective antitumor drug but can contribute to cardiomyocyte injuries. We aimed to dissect the mechanism of the HMOX1/CTGF axis in DOX-induced cardiomyocyte injury, mitochondrial dysfunction, and ferroptosis.
Methods
Bioinformatics analysis was conducted to retrieve differentially expressed genes in a DOX-induced mouse model. Mouse cardiomyocytes, HL-1 cells, were induced with l µM DOX, after which gain- or loss-of-function assays were applied. CCK-8, fluorescent probe assay, flow cytometry, and corresponding kits were employed to detect cell viability, ROS levels, mitochondrial membrane potential and cell apoptosis, and GSH and Fe2+ contents, respectively. qRT-PCR or Western blot assay was adopted to test HMOX1, CTGF, BCL-2, Caspase3, Cleaved-Caspase3, and GPX4 expression.
Results
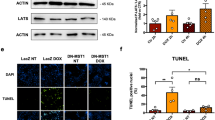
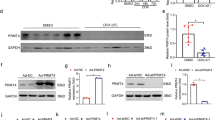
Bioinformatics analysis showed that HMOX1 and CTGF were highly expressed in DOX-induced mice and correlated with each other. Also, HMOX1 and CTGF expression was high in HL-1 cells after DOX treatment, along with an obvious decrease in cell viability and GSH and GPX4 expression, an increase in ROS levels, apoptosis, and Fe2+ contents, and mitochondrial membrane potential dysfunction or loss. HMOX1 or CTGF silencing diminished cell apoptosis, Cleaved-Caspase3 expression, Fe2+ contents, and ROS levels, enhanced cell viability and the expression of GSH, GPX4, and BCL-2, and recovered mitochondrial membrane potential in DOX-induced HL-1 cells. Nevertheless, the effects of HMOX1 silencing on the viability, apoptosis, ferroptosis, and mitochondrial dysfunction of DOX-induced HL-1 cells were counteracted by CTGF overexpression.
Conclusions
In conclusion, HMOX1 silencing decreased CTGF expression to alleviate DOX-induced injury, mitochondrial dysfunction, and ferroptosis of mouse cardiomyocytes.




Similar content being viewed by others
References
Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 2014;10:853–8.
Cagel M, Grotz E, Bernabeu E, Moretton MA, Chiappetta DA. Doxorubicin: nanotechnological overviews from bench to bedside. Drug Discov Today. 2017;22:270–81.
Yao CX, Shi JC, Ma CX, Xiong CJ, Song YL, et al. EGF protects cells against dox-induced growth arrest through activating cyclin d1 expression. J Cell Biochem. 2015;116:1755–65.
Al-Malky HS, Al Harthi SE, Osman AM. Major obstacles to doxorubicin therapy: cardiotoxicity and drug resistance. J Oncol Pharm Pract. 2020;26:434–44.
Prathumsap N, Shinlapawittayatorn K, Chattipakorn SC, Chattipakorn N. Effects of doxorubicin on the heart: from molecular mechanisms to intervention strategies. Eur J Pharmacol. 2020;866: 172818.
Liu Y, Zeng L, Yang Y, Chen C, Wang D, Wang H. Acyl-CoA thioesterase 1 prevents cardiomyocytes from doxorubicin-induced ferroptosis via shaping the lipid composition. Cell Death Dis. 2020;11:756.
Hu C, Zhang X, Song P, Yuan YP, Kong CY, et al. Meteorin-like protein attenuates doxorubicin-induced cardiotoxicity via activating cAMP/PKA/SIRT1 pathway. Redox Biol. 2020;37: 101747.
Wang AJ, Zhang J, Xiao M, Wang S, Wang BJ, et al. Molecular mechanisms of doxorubicin-induced cardiotoxicity: novel roles of sirtuin 1-mediated signaling pathways. Cell Mol Life Sci. 2021;78:3105–25.
Schipper HM, Song W, Tavitian A, Cressatti M. The sinister face of heme oxygenase-1 in brain aging and disease. Prog Neurobiol. 2019;172:40–70.
Singh N, Ahmad Z, Baid N, Kumar A. Host heme oxygenase-1: friend or foe in tackling pathogens? IUBMB Life. 2018;70:869–80.
Menon AV, Liu J, Tsai HP, Zeng L, Yang S, et al. Excess heme upregulates heme oxygenase 1 and promotes cardiac ferroptosis in mice with sickle cell disease. Blood. 2022;139:936–41.
Chen C, Shen H, Huang Q, Li Q. The circular rna cdr1as regulates the proliferation and apoptosis of human cardiomyocytes through the mir-135a/hmox1 and mir-135b/hmox1 axes. Genet Test Mol Biomark. 2020;24:537–48.
Meyer N, Zielke S, Michaelis JB, Linder B, Warnsmann V, et al. AT 101 induces early mitochondrial dysfunction and HMOX1 (heme oxygenase 1) to trigger mitophagic cell death in glioma cells. Autophagy. 2018;14:1693–709.
Ramazani Y, Knops N, Elmonem MA, Nguyen TQ, Arcolino FO, et al. Connective tissue growth factor (CTGF) from basics to clinics. Matrix Biol. 2018;68–69:44–66.
Vainio LE, Szabo Z, Lin R, Ulvila J, Yrjola R, et al. Connective tissue growth factor inhibition enhances cardiac repair and limits fibrosis after myocardial infarction. JACC Basic Transl Sci. 2019;4:83–94.
Valentijn FA, Knoppert SN, Pissas G, Rodrigues-Diez RR, Marquez-Exposito L, et al. CCN2 aggravates the immediate oxidative stress-dna damage response following renal ischemia-reperfusion injury. Antioxidants (Basel). 2021;10:2020.
Lai WT, Li YJ, Wu SB, Yang CN, Wu TS, et al. Connective tissue growth factor decreases mitochondrial metabolism through ubiquitin-mediated degradation of mitochondrial transcription factor A in oral squamous cell carcinoma. J Formos Med Assoc. 2018;117:212–9.
Kuo CY, Chiu V, Hsieh PC, Huang CY, Huang SJ, et al. Chrysophanol attenuates hepatitis B virus X protein-induced hepatic stellate cell fibrosis by regulating endoplasmic reticulum stress and ferroptosis. J Pharmacol Sci. 2020;144:172–82.
Bauer B, Liedtke D, Jarzina S, Stammler E, Kreisel K, et al. Exploration of zebrafish larvae as an alternative whole-animal model for nephrotoxicity testing. Toxicol Lett. 2021;344:69–81.
Aung LHH, Li R, Prabhakar BS, Li P. Knockdown of Mtfp1 can minimize doxorubicin cardiotoxicity by inhibiting Dnm1l-mediated mitochondrial fission. J Cell Mol Med. 2017;21:3394–404.
Wenningmann N, Knapp M, Ande A, Vaidya TR, Ait-Oudhia S. Insights into doxorubicin-induced cardiotoxicity: molecular mechanisms, preventive strategies, and early monitoring. Mol Pharmacol. 2019;96:219–32.
Tadokoro T, Ikeda M, Ide T, Deguchi H, Ikeda S, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020. https://doi.org/10.1172/jci.insight.132747.
Cheng X, Liu D, Song H, Tian X, Yan C, Han Y. Overexpression of kininogen-1 aggravates oxidative stress and mitochondrial dysfunction in dox-induced cardiotoxicity. Biochem Biophys Res Commun. 2021;550:142–50.
Zheng X, Zhong T, Ma Y, Wan X, Qin A, et al. Bnip3 mediates doxorubicin-induced cardiomyocyte pyroptosis via caspase-3/GSDME. Life Sci. 2020;242: 117186.
Kitakata H, Endo J, Matsushima H, Yamamoto S, Ikura H, et al. MITOL/MARCH5 determines the susceptibility of cardiomyocytes to doxorubicin-induced ferroptosis by regulating GSH homeostasis. J Mol Cell Cardiol. 2021;161:116–29.
Zhao L, Qi Y, Xu L, Tao X, Han X, et al. MicroRNA-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting Nrf2 and Sirt2. Redox Biol. 2018;15:284–96.
Kiraz Y, Adan A, Kartal Yandim M, Baran Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016;37:8471–86.
Song S, Chu L, Liang H, Chen J, Liang J, et al. Protective effects of dioscin against doxorubicin-induced hepatotoxicity via regulation of sirt1/foxo1/nf-kappab signal. Front Pharmacol. 2019;10:1030.
Bernard A, Chevrier S, Beltjens F, Dosset M, Viltard E, et al. Cleaved caspase-3 transcriptionally regulates angiogenesis-promoting chemotherapy resistance. Cancer Res. 2019;79:5958–70.
Yang B, Chen Y, Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem Rev. 2019;119:4881–985.
Zhang Q, Wu G, Guo S, Liu Y, Liu Z. Effects of tristetraprolin on doxorubicin (adriamycin)-induced experimental kidney injury through inhibiting IL-13/STAT6 signal pathway. Am J Transl Res. 2020;12:1203–21.
Sakamuru S, Attene-Ramos MS, Xia M. Mitochondrial membrane potential assay. Methods Mol Biol. 2016;1473:17–22.
Yin J, Guo J, Zhang Q, Cui L, Zhang L, et al. Doxorubicin-induced mitophagy and mitochondrial damage is associated with dysregulation of the PINK1/parkin pathway. Toxicol In Vitro. 2018;51:1–10.
Stamenkovic A, O’Hara KA, Nelson DC, Maddaford TG, Edel AL, et al. Oxidized phosphatidylcholines trigger ferroptosis in cardiomyocytes during ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2021;320:H1170–84.
Ursini F, Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med. 2020;152:175–85.
Wang J, Deng B, Liu Q, Huang Y, Chen W, et al. Pyroptosis and ferroptosis induced by mixed lineage kinase 3 (MLK3) signaling in cardiomyocytes are essential for myocardial fibrosis in response to pressure overload. Cell Death Dis. 2020;11:574.
Ahmed AZ, Mumbrekar KD, Satyam SM, Shetty P, D’Souza MR, Singh VK. Chia seed oil ameliorates doxorubicin-induced cardiotoxicity in female wistar rats: an electrocardiographic. Biochem Histopathol Approach Cardiovasc Toxicol. 2021;21:533–42.
Zhang H, Wang Z, Liu Z, Du K, Lu X. Protective effects of dexazoxane on rat ferroptosis in doxorubicin-induced cardiomyopathy through regulating HMGB1. Front Cardiovasc Med. 2021;8: 685434.
Deng X, Liang C, Qian L, Zhang Q. miR-24 targets HMOX1 to regulate inflammation and neurofunction in rats with cerebral vasospasm after subarachnoid hemorrhage. Am J Transl Res. 2021;13:1064–74.
Fang X, Wang H, Han D, Xie E, Yang X, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. 2019;116:2672–80.
Qin D, Yue R, Deng P, Wang X, Zheng Z, et al. 8-Formylophiopogonanone B antagonizes doxorubicin-induced cardiotoxicity by suppressing heme oxygenase-1-dependent myocardial inflammation and fibrosis. Biomed Pharmacother. 2021;140: 111779.
Meng Z, Liang H, Zhao J, Gao J, Liu C, et al. HMOX1 upregulation promotes ferroptosis in diabetic atherosclerosis. Life Sci. 2021;284: 119935.
Moreno-Navarrete JM, Ortega F, Rodriguez A, Latorre J, Becerril S, et al. HMOX1 as a marker of iron excess-induced adipose tissue dysfunction, affecting glucose uptake and respiratory capacity in human adipocytes. Diabetologia. 2017;60:915–26.
Stephany HA, Strand DW, Ching CB, Tanaka ST, Milne GL, et al. Chronic cyclic bladder over distention up-regulates hypoxia dependent pathways. J Urol. 2013;190:1603–9.
Chen L, Ji Q, Zhu H, Ren Y, Fan Z, Tian N. miR-30a attenuates cardiac fibrosis in rats with myocardial infarction by inhibiting CTGF. Exp Ther Med. 2018;15:4318–24.
Ouyang W, Fu S, Zhao X, Su S, Zhang J, et al. Recombinant human endostatin combined with radiotherapy promotes cardiomyocyte apoptosis in rats via TGFbeta1/Smads/CTGF signaling pathway. BMC Cardiovasc Disord. 2022;22:97.
De Francesco EM, Rocca C, Scavello F, Amelio D, Pasqua T, et al. Protective role of gper agonist g-1 on cardiotoxicity induced by doxorubicin. J Cell Physiol. 2017;232:1640–9.
Behnes M, Brueckmann M, Lang S, Weiss C, Ahmad-Nejad P, et al. Connective tissue growth factor (CTGF/CCN2): diagnostic and prognostic value in acute heart failure. Clin Res Cardiol. 2014;103:107–16.
Chi H, Feng H, Shang X, Jiao J, Sun L, et al. Circulating connective tissue growth factor is associated with diastolic dysfunction in patients with diastolic heart failure. Cardiology. 2019;143:77–84.
Waza AA, Hamid Z, Bhat SA, Shah NUD, Bhat M, Ganai B. Relaxin protects cardiomyocytes against hypoxia-induced damage in in-vitro conditions: Involvement of Nrf2/HO-1 signaling pathway. Life Sci. 2018;213:25–31.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no relationships that could be construed as a conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qian, J., Wan, W. & Fan, M. HMOX1 silencing prevents doxorubicin-induced cardiomyocyte injury, mitochondrial dysfunction, and ferroptosis by downregulating CTGF. Gen Thorac Cardiovasc Surg 71, 280–290 (2023). https://doi.org/10.1007/s11748-022-01867-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-022-01867-7