Abstract
Cholesterol has been used to monitor artifact generation. Stability differences among cholesterol oxide products (COPs) and cholesterol in thermal and alkaline conditions are theorized. Thus, use of cholesterol may be unsuitable for detection of artifacts generated from COPs. Stability of cholesterol was compared to that of 7-ketocholesterol (7-keto) and β-sitosterol (βS) under various thermal and alkaline saponification conditions: 1 M methanolic KOH for 18 h at 24 °C (1 M18hr24°C, Control), 18 h at 37 °C (1M18hr37°C), 3 h at 45 °C (1M3hr45°C), and 3.6 M methanolic KOH for 3 h at 24 °C (3.6M3hr24°C). Trends indicated that cholesterol in solution was more stable than 7-keto under all conditions. Compared to βS, cholesterol was more stable under all conditions except for 1M18hr37°C for which stabilities were similar. Compounds were more labile in heat than alkalinity. Poor recoveries of 7-keto during cold saponification with high alkalinity were attributed to alkaline instability. 7-Keto, less stable than cholesterol, should be used to monitor artifact generation during screening of various methods that include thermal and alkaline conditions. In a preliminary analysis of turkey meat, more 3,5-7-one was generated from spiking with cholesterol than with 7-keto.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
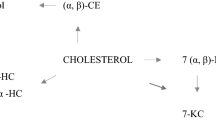
Cholesterol undergoes free radical initiation resulting in cholesterol hydroperoxides that decompose into various cholesterol oxide products (COPs). Initial COPs can remain unaffected or undergo further reactions, resulting in second and third generation COPs [1].
Artifacts are generated by the breakdown of cholesterol or COPs caused by various conditions of analysis. Cholesta-3,5-diene-7-one (3,5-7-one), resulting from breakdown of 7-ketocholesterol (7-keto), is thought to exist only as an artifact. Cholestane-3β,5α,6β-triol (triol) can exist both as a COP and as an artifact.
Many discrepancies among quantities of COPs found in similar samples [2–6] may be due to artifacts [5, 7, 8] and incomparable methodologies. Various COPs also exhibit different susceptibilities to breakdown during storage and analysis [9]. For instance, cholestane-3β,5α,6β-triol (triol) and 7-keto exhibited instability over time when stored at −20, 4 and 22 °C while solutions of cholest-3,5-dien-7-one (3,5-7-one) and 5α-cholestane (5αC) remained stable over a 7 day period at these temperatures [9]. Changing of injection liners and number of injections were often complicating factors in analyses [9].
Saponification conditions can produce artifacts. Most saponifications are performed at room temperature to lessen the generation of artifacts caused by elevated thermal conditions. Park et al. [8] demonstrated 7-keto breakdown and 3,5-7-one generation as saponification temperature increased. Dzeletovic et al. [2] reported decomposition of 7-keto at temperatures greater than 22 °C. In addition, alkaline concentration used to saponify can affect the stability of 7-keto. Park et al. [8] reported a 10% loss of 7-keto with generation of 3,5-7-one during a 3.6 M (~20%) methanolic KOH saponification at 22 °C for 3 h. Lampi et al. [10] recovered only ~54% of 7-keto following overnight saponification with saturated KOH at 25 °C in a shaking water bath with nitrogen purging.
Artifacts monitoring and/or reporting may not occur. A common method of monitoring artifact generation is the analysis of labeled or unlabeled standard cholesterol [7, 11–15]. Spiking with an optimized level of purified cholesterol was the recommended method following the Lausanne Workshop [5]. Several investigators have advocated and used multiple standards of labeled COPs and fluorinated internal standards of COPs to monitor artifact generation [2, 6, 11, 16–19]. Dzeletovic et al. [2] considered use of multiple deuterium-labeled internal standards as the preferred method for sensitivity, specificity and recovery correction, but noted drawbacks of laborious sample preparation and a limited number of samples.
Generally, breakdown of 7-keto during saponification is of greater concern than the breakdown of cholesterol. Because the goal of many analyses is quantification of COPs, rather than solely cholesterol, it is important to monitor breakdown of COPs as well. Unlabeled or labeled cholesterol as a monitoring standard cannot account for the loss or artifact generation of COPs unless it exhibits the same or less stability as the COPs of interest. If cholesterol is more stable than various COPs, it is a poor model and monitor of artifact generation.
Phytosterols, similar in structure to cholesterol, undergo oxidation and yield phytosterol oxide products (POPs). The same spate of oxidation products (7-ketophytosterols, 7-hydroxyphytosterols, and trihydroxyphytosterols) are produced from phytosterols. Analyses of phytosterols and POPs are increasingly common as consumption of phytosterols is investigated in terms of health effects [20]. With numerous phytosterols in existence and more POPs than COPs, analysis of the former is an even greater challenge. An additional complication is the lack of commercial phytosterol and standards of POPs. Artifact generation of phytosterols and POPs occurs as it does for cholesterol and COPs; therefore, artifact monitoring of phytosterols and POPs is essential.
There have been few investigations contrasting the use of cholesterol and COPs in monitoring artifact generation. These comparisons are needed because not only has cholesterol been used to monitor artifact generation during analysis of COPs, it has been used to that end during analysis of POPs as well [21, 22].
Because the goal of many analyses is the separate or simultaneous quantification of COPs and POPs, it is especially important to examine the breakdown of specific compounds under varying conditions of analysis such as saponification. It is hypothesized that cholesterol exhibits greater stability during thermal and alkaline saponification conditions compared to 7-keto. Thus, the goal of the present work was to quantify artifact generation from cholesterol, 7-keto, and β-sitosterol (βS) during saponification using thermal and/or alkaline conditions. A preliminary experiment to ascertain if spiking turkey meat with cholesterol and 7-keto generated artifacts during saponification under specific conditions was conducted. Additionally, ramifications of differences in stability of cholesterol, COPs, phytosterols, and POPs for monitoring artifact generation are discussed.
Experimental Section
Materials
Cholesterol and βS were obtained from Sigma (St. Louis, MO). 7-keto; 3,5-7-one; and cholest-5-ene-7-one (5-ene-7-one) were obtained from Steraloids (Newport, RI). Toluene (Omni-Solv) was from EM Science (EMD Chemicals Inc, Gibbstown, NJ). Stable, septum-sealed anhydrous ethyl ether, potassium hydroxide, and methanol were from EM Science (EMD Chemicals Inc, Gibbstown, NJ). Anhydrous sodium sulfate and anhydrous pyridine were products of Fisher Scientific (Pittsburgh, PA) and Aldrich (Milwaukee, WI), respectively. Sylon BTZ was obtained from Supelco (Bellafonte, PA). Silanized vials and inserts were purchased from Fisher Scientific (Pittsburgh, PA). Silica solid phase extraction (SPE) cartridges were procured from Phenomenex (Torrance, CA). Turkey thighs (2–3 days post mortem) were purchased from a local grocery store.
In all methods employed, artifact limiting conditions included low light, nitrogen flushing and drying under nitrogen at room temperature (22 ± 2 °C). No antioxidants were used so that the actual effects of each saponification condition could be assessed. To avoid the possibility of peroxide formation, new septum-sealed containers (500 mL) of anhydrous ethyl ether were opened and used on the day of each analysis.
Saponification of Standards in Solution
Saponification and Extraction
Previously in our work, 19-hydroxycholesterol, a common internal standard, was found to co-elute with 3,5-7-one [23] and 5α-cholestane was reported to yield interferences in analysis of dark chicken meat [24, 25]; therefore, 5-ene-7-one was used as an instrument internal standard. 2.0 mg/mL stock solution of 5-ene-7-one in toluene was prepared; 0.5 mg/mL dilutions were made. Individual stock solutions of cholesterol, 7-keto and βS in toluene were prepared and diluted to 0.1 mg/mL. In triplicate, 450 μL of a single COP (0.1 mg/mL) solution was added to a silanized, amber vial and dried under nitrogen to completeness.
Dried samples were re-dissolved in 5 mL anhydrous ethyl ether and saponified in the dark in a shaking water bath under one of the following conditions: 1 M methanolic KOH for 18 h at 24 °C (1M18hr24°C, Control), 18 h at 37 °C (1M18hr37°C), 3 h at 45 °C (1M3hr45°C), or 3.6 M methanolic KOH for 3 h at 24 °C (3.6M3hr24°C). 1M18hr24°C, cold saponification, was used as the control because it is regarded by many researchers as appropriate for generation of minimal artifacts during analysis. 1 M KOH was considered low alkaline while 3.6 M was considered high. Saponifications at 24 °C were considered “cold” while 37 and 45 °C were considered medium and high temperature, respectively. Each day, triplicate samples of the three compounds from a single saponification condition were analyzed and each condition was repeated for a total of six samples per compound.
Using the method of Guardiola et al. [13] with slight modification, COPs were extracted by addition of 15 mL double distilled water followed by 10 mL of anhydrous ethyl ether and vigorous shaking. The organic phase was extracted and removed to a silanized amber vial and flushed with nitrogen. After extraction of the aqueous phase twice more with anhydrous ethyl ether, organic phases were pooled. Three ethyl ether extractions were performed.
To pooled organic extracts, 5 mL of 0.5 M aqueous KOH were added, flushed with nitrogen, and vigorously shaken. Pooled organic extracts were washed twice with 5 mL of double distilled water for a total of three washes (1:2 KOH and water) and flushed with nitrogen. Washed organic phases were filtered through 30 g of anhydrous sodium sulfate in Whatman no. 1 filters and collected in a round bottom flask. Anhydrous sodium sulfate was washed with 6 mL of anhydrous ethyl ether and collected in the same round bottom flask and flushed with nitrogen. All, but 1–2 mL of extract was dried in a rotary vacuum evaporator at room temperature. Remaining extract was transferred to a silanized gas chromatograph (GC) vial and dried under a stream of nitrogen. The round-bottom flask was rinsed with 2 mL of anhydrous ethyl ether, which was collected and added to the GC vial and dried to completeness under nitrogen.
Derivatization and Conditions for GC Analysis
Dried samples were derivatized with 50 μL of anhydrous pyridine and 50 μL of Sylon BTZ after room temperature incubation for 30 min. Derivatized samples were transferred to silanized GC inserts containing 50 μL of 5-ene-7-one in toluene, the instrument internal standard, and analyzed by gas chromatography–flame ionization detection (GC–FID) with a 30 m, 0.25 mm × 0.25 µm DB-5 ms column. The carrier gas was helium. The injection temperature was 290 °C, detector temperature was 330 °C. The split ratio was set at 30:1. The temperature program was 100 °C for 1 min, 100–260 °C with a ramp of 20 °C/min, 260–273 °C with a ramp of 5 °C/min, and 273–275 °C with a ramp of 0.1 °C/min. Hydroxyl groups were derivatized to trimethylsilyl ethers (tms ethers) whereas keto groups were not derivatized. Under the conditions and reagents used, 3,5-7-one and 5-ene-7-one did not have derivatizable functional groups. Standard curves of each compound were used to calculate data for results discussed below.
Preliminary Experiment: Analysis of Control and Spiked Turkey Meat
Turkey thighs were de-boned and finely ground in a Cuisinart food processor. Folch lipid extractions were performed on triplicate aliquots (3 g) of control ground turkey meat and then spiked with 100 mg of either cholesterol or 7-keto. Lipid extracts were re-dissolved in anhydrous ethyl ether and exposed to saponification conditions (1M18hr24°C and 1M3hr45°C) in a shaking water bath. Extractions were performed as noted above.
Saponified extracts were subjected to solid phase extraction (SPE) [13] with silica cartridges using hexane and a series of hexane/ethyl ether solutions with increasing polarity (95:5, 90:10, 80:20 v/v). COPs were eluted with acetone/methanol (60:20 v/v) and collected in a round-bottom flask. Fractions were dried in a rotary vacuum evaporator at room temperature. Remaining samples (1–2 mL) were transferred to silanized GC vials. The round-bottom flask was rinsed with 2 mL of anhydrous ethyl ether, which was collected and added to the GC vial and dried to completeness under nitrogen. GC conditions were those delineated above.
Experimental Design and Statistical Analysis
Data for the primary experiment were analyzed as a 4 (tms ethers of cholesterol, 7-keto, and βS; and 3, 5-7-one) × 3 (triplicate samples) × 4 (saponification conditions as 1M18hr24°C, 1M18hr37°C, 1M3hr45°C, and 3.6M3hr24°C) × 2 (replications) factorial design using ANOVA. The Welch two-sample t test was used to separate means at P ≤ 0.10. Data for results of the preliminary experiment were not statistically analyzed; averages of three samples were calculated for an initial comparison.
Results
Means of six samples for each condition and compound had high standard deviations, most likely resulting from extensive handling and manipulation of small quantities of samples. While the standard deviations resulted in no significant differences among means, the reported trends are highly informative and can be used to initiate further investigations to reduce artifact generation. Percentages discussed below were calculated relative to that for the Control, 1M18hr24°C (100%). As noted above, many researchers regard 1M18hr24°C (cold saponification) as appropriate for generation of minimal artifacts during analysis.
COPs in Solution
Cholesterol
Retention of cholesterol was at least 100% relative to control conditions of 1M18hr24°C and under all saponification conditions tested except for 1M3hr45°C, at which 73% was retained (Table 1).
7-Ketocholesterol
Under conditions with temperatures greater than 24 °C, 7-keto was retained at only 53 and 49% relative to the Control, suggesting possible susceptibility to medium and high temperatures (Table 1). 7-Keto was retained at 71% at 3.6M3hr24°C relative to 1M18hr24°C revealing likely alkaline instability as well.
The degradation product of 7-keto, 3,5-7-one, was initially present as a contaminant in the standard solution of 7-keto prior to saponification and analysis. The percentage of 3,5-7-one in the 7-keto standard solution was subtracted from the percentage detected in the samples so that results are presented as corrected values. The corrected 3,5-7-one value at 1M18hr24°C was set at 100% and the other conditions were calculated relative to this value. Under saponification conditions with increased temperature (1M18hr37°C and 1M3hr45°C), 3,5-7-one was 398 and 344% greater, respectively, than under the 100% control conditions. At the increased alkaline concentration of 3.6M3hr24°C, 59% less 3,5-7-one was present compared to the Control.
β-Sitosterol
Relative to the Control, βS seemed stable at 1M18hr37°C, but was retained at only 59% at 1M3hr45°C (Table 1). 92% of βS was retained under the elevated alkaline condition of 3.6M3hr24°C relative to 1M18hr24°C (Table 1).
COPs in Turkey Meat
While the major focus of our research was to examine the often false assumptions about conditions for artifact production using standard compounds, it was important to conduct an initial examination to ascertain the level of oxides produced in a food sample containing cholesterol and COPs. When ground turkey meat was analyzed after saponification at 1M18hr24°C, only cholesterol was detected (Fig. 1a). Following saponification at 1M3hr45°C, both cholesterol and 7-keto were detected, suggesting that these saponification conditions generated 7-keto as an artifact. 3,5-7-One was not detected at 1M18hr24°C or 1M3hr45°C (Fig. 1a).
Average (3 samples) peak area ratio of COP in a turkey meat (3 g), b spiked with cholesterol (100 mg), and c spiked with 7-ketocholesterol (100 mg). Solid shading indicates saponification conditions of 1M18hr24°C and vertical stripes indicate 1M3hr45°C. Cholest-5-ene-7-one (5-ene-7-one) was used as an internal standard
Spiking with cholesterol produced a low amount of 7-keto at 1M3hr45°C (Fig. 1b). When ground turkey meat was spiked with 7-keto and saponified under conditions of 1M3hr45°C, the amount of cholesterol detected decreased and more 7-keto was recovered compared to conditions of 1M18hr24°C (Fig. 1c). For spiking with 7-keto, 3,5-7-one was generated as an artifact during both saponification conditions (Fig. 1c).
Discussion
COPs in Solution
Cholesterol
While cholesterol was stable at the increased temperature of 37 °C, it seemed heat labile at 45 °C with 1M KOH for the short time of 3 h. Lack of decomposition at 1M18hr37°C and 3.6M3hr24°C may demonstrate high alkaline stability at medium temperature.
7-Ketocholesterol
Heat instability of 7-keto (47% retained) at 1M18hr37°C as compared to 1M18hr24°C confirmed findings reported by other investigators[3, 8]. Percent retention of 7-keto, similar at 1M18hr37°C (53%) and 1M3hr45°C (49%), showed that the rate of decomposition at the higher temperature/short time was greatly accelerated. This finding is in agreement with that of Park et al. [8] who suggested that small temperature changes can greatly influence 7-keto degradation. Retentions of 7-keto at 71% for 3.6M3hr24°C relative to 1M18hr24°C, indicated more sensitivity to high alkalinity than prolonged saponification. As noted above, Lampi et al. [10] realized that heat caused 7-keto destruction and conversion to 3,5-7-one, but failed to recognize alkaline instability as a likely cause of low 7-keto recovery during cold saponification with saturated KOH.
Generation of 3,5-7-one from 7-keto was expected with increased temperature and alkalinity. However, it seemed that more 3,5-7-one was generated at 1M18hr37°C than at 1M3hr45°C. This may have been caused by prolonged saponification at the medium temperature. The loss of 7-keto and considerably less generation of 3,5-7-one at 3.6M3hr24°C as compared to the Control was contradictory to reported observations [2–6]. It should be noted that even though the trend showed that more 3,5-7-one was generated at medium temperature as compared to the high alkaline condition, alkaline concentration remains an important and often overlooked source of artifact generation.
In general, our relative percentages for retention are greater than values observed and predicted by Park et al. [8]. Percentages presented in the present study are relative to 1M18hr24°C in order to compare retention of compounds under the various saponification conditions whereas Park et al.’ loss percentages are recovery values. However, it is informative to compare the values. In our study, 47% of 7-keto was lost at 37 °C relative to that at 24 °C. This is greater than the 30% loss of 7-keto at the same temperature of saponification predicted by Park et al. [8] based on calculations by the Arrhenius equation. Our findings indicated a loss of 51% of 7-keto at 1M3hr45°C, relative to 1M18hr24°C. Park et al. [8] found roughly 10% loss of 7-keto at 45 °C for about 2 h. For 3.6M3hr24°C saponification, our results showed 29% loss of 7-keto compared to the reported ~10% loss of 7-keto at 20% methanolic KOH (~3.6 M) for 3 h at 22 °C by Park et al. [8]. While actual values from our study differ from those of Park et al. our trends show losses under similar conditions.
β-Sitosterol
Compared to the Control, βS seemed stable at the elevated temperature of 37 °C, slightly less stable at 3.6M3hr24°C and the least stable at 45 °C. Like cholesterol and 7-keto, βS was considered more labile at the high temperature than at high alkalinity.
Comparisons of Compounds
Cholesterol was more stable under all conditions compared to 7-keto. Compared to βS, cholesterol was more stable under all conditions, however at 1M18hr37°C both compounds had similar relative recoveries. Cholesterol exhibited greater temperature stability and alkaline stability than βS and 7-keto. βS was more stable under all conditions than 7-keto.
1M18hr24°C saponification conditions were hypothesized and revealed to yield the least loss of 7-keto compared to all other conditions. For all compounds, there was more retention at 3.6M3hr24°C than at 1M3hr45°C indicating a greater susceptibility to decomposition at 45 °C than at a high alkalinity of 3.6 M. While more 3,5-7-one was generated at higher temperatures than at high alkalinity, alkaline saponification concentration must still be recognized as an important source of possible artifact generation.
COPS in Turkey Meat
In uncooked ground turkey meat, negligible amounts of COPs were expected for 1M18hr24°C [3]. Compared to 1M18hr24°C, 1M3hr45°C probably generated artifacts. Again, as noted above for cholesterol and 7-keto in solution at 1M3hr45°C, the unexpected loss of cholesterol occurred concomitantly with generation of 7-keto in control and spiked turkey meat under these conditions.
At 1M3hr45°C, cholesterol spiked turkey yielded both 7-keto and 3,5-7-one as artifacts rather than just 7-keto. In 7-keto spiked meat, it was more difficult to isolate artifact generation because cholesterol was naturally present and breaking down into 7-keto at the highest temperature. It was expected that more 3,5-7-one would be generated at 1M3hr45°C than at 1M18hr24°C as was found in solutions of 7-keto above, however, the quantity of 3,5-7-one was similar for 7-keto spiked meat under both conditions. This finding is aberrant to the theory of heat instability for 7-keto relative to cholesterol. It is possible that the matrix interfered with the effect of temperature for 1M3hr45°C. Extending work on turkey, other types of meat, and various matrices could help to clarify our findings.
Ramifications for Artifact Monitoring of COPs
Trends for differences in stability among cholesterol and COPs are useful in terms of initial artifact monitoring for methods using thermal and alkaline saponifications, but caution is warranted. From our results for COPs in solution, if cholesterol alone were used to monitor loss or artifact generation during saponification at 1M18hr37°C, no loss of cholesterol would be detected. Therefore, a method could be validated for use, even though there would be lost of 7-keto with concomitant overestimation of its quantity and generation of 3,5-7-one as an artifact. Thus, in solution, cholesterol is too stable to monitor artifact generation from some COPs; its use as a monitor of loss and artifact generation would be inaccurate for compounds less stable than itself. Likewise, βS as a monitor would overestimate quantities of 7-keto and underestimate cholesterol. In meat and other food matrices, results are more complicated and ramifications for artifact monitoring are less clear.
While labeled and unlabeled cholesterol as a monitor of artifact generation is used by several researchers, it can only monitor breakdown of cholesterol and COPs that are as stable, or more stable, than cholesterol, and not every COP of interest. Some investigators advocate the use of individually labeled COPs to monitor artifact generation and for recovery purposes. However, use of “deuterium labeled cholesterol or COPs for monitoring cannot be expected to come into common use in food laboratories” [5] and are “inappropriate for routine use” [26]. Thus, it is proposed that when stabilities of COPs and cholesterol for required thermal and alkaline conditions are determined, the least stable COP should be used to monitor artifact generation. While not accounting for loss of each analyte, an inexpensive and quick marker, like 7-keto or another appropriate COP, could monitor artifact generation for method development and comparison studies. Further investigations on the stabilities of more COPs during analysis are needed.
Ramifications for Artifact Monitoring of POPs
Although probably not most appropriate, cholesterol has been used to monitor artifact generation during analysis of POPs. Louter [27] used a mixture of phytosterols to monitor artifact generation during saponification and deuterated cholesterol to monitor the entire procedure by spiking various types of samples which modeled stages of the method. Only deuterated 7-keto was found, however, it was initially present as a contaminant in deuterated cholesterol. This is noteworthy because saponification consisted of 2 M, rather than 1 M, ethanolic KOH for 18 h at ambient temperature in the dark. Moreover, unlabeled COPs have been used in POP analysis of vegetable oils, mostly for their similar polarity to POPs in terms of recovery. Bortolomeazzi et al. [22] used 7α-hydroxycholesterol as an internal standard for hydroxy POPs and 7-ketocholesterol for POPs with a 7-keto functional group. They reported that an added benefit of 7-keto as an internal standard was its ability to highlight possible breakdown behavior of POPs during cold saponification. However, because a 2 M ethanolic KOH cold saponification was performed overnight, the stability of 7-keto under this alkalinity in the matrix used would have to be investigated prior to its use for purposes of quantification. If the internal standard used broke down during analysis, reported recoveries would be erroneous. Previously, 2 M KOH cold saponifications in milk powder, egg products, and Dutch mixed diets along with chicken meat have resulted in greater than 60% loss of 7-keto [28]. Multiple individually labeled POPs have also been used as internal standards and artifact monitors for analysis of POPs in serum and lipid infusions [20]. As with analysis of COPs in food matrices, this is likely the most accurate method for artifact monitoring and recovery; however, work required was extensive because the POPs standards had to be synthesized and labeled.
Conclusion
We propose that using cholesterol to monitor saponification conditions for artifact generation from COPs and POPs may result in possible under-reporting of 7-keto, over-reporting of 3,5-7-one, and unwittingly, continued use of methods that yield artifacts. Although extreme caution is advised, the differences in stabilities among cholesterol, 7-keto, and βS could be useful in terms of artifact monitoring. Due to the large number of phytosterols, numerous POPs resulting for each phytosterol and the lack of commercial POPs standards, determination and use of representative least stable POPs as markers may be a more effective method for monitoring phytosterol oxide artifact generation in very limited situations.
References
Smith LL (1987) Cholesterol autoxidation 1981–1986. Chem Phys Lipids 44:87–126
Dzeletovic S, Breuer O, Lund E, Diczfalusy U (1995) Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal Biochem 225:73–80
Paniangvait P, King AJ, Jones AD, German BG (1995) Cholesterol oxides in foods of animal origin. J Food Sci 60:1159–1174
Dutta PC, Caboni MF, Diczfalusy U, Dionisi F, Dzeletovic S, Grandgirard A, Guardiola F, Kumpulainen J, Lebovics VK, Pihlava JM, Rodriguez-Estrada MT, Ulberth F (1999) In: Kumpulainen JT, Salonen JT (eds) Natural antioxidant and anticarcinogens in nutrition, health and disease. Royal Society of Chemistry, Cambridge, pp 309–315
Appelqvist LA, Addis P, Bjorkhem I, Bosset JO, Caboni MF, Dutta P, Grandgirard A, Guardiola F, Hau LB, Nielsen JH, Hugget A, Hwang LS, Kumpulainen J, McCluskey S, Ohshima T, Przbylski R, Sevanian A, Yan P (2004) Harmonization of methods for analysis of cholesterol oxides in foods–the first portion of a long road toward standardization: Interlaboratory study. J AOAC Int 87:511–519
Leoni V, Lutjohann D, Masterman T (2005) Levels of 7-oxocholesterol in cerebrospinal fluid are more than one thousand times lower than reported in multiple sclerosis. J Lipid Res 46:191–195
Rose-Sallin C, Huggett AC, Bosset JO, Tabacchi R, Fay LB (1995) Quantification of cholesterol oxidation products in milk powders using [2H7]cholesterol to monitor cholesterol autoxidation artifacts. J Agric Food Chem 43:935–941
Park PW, Guardiola F, Park SH, Addis PB (1996) Kinetic evaluation of 3-β-hydroxycholest-5-en-7-one (7-ketocholesterol) stability during saponification. J Am Oil Chem Soc 73:623–629
Busch TJ, King AJ (2009) Artifact generation and monitoring in analysis of cholesterol oxide products. Anal Biochem 399:1–14
Lampi A-M, Juntunen L, Toivo J, Piironen V (2002) Determination of thermo-oxidation products of plant sterols. J Chromatogr B 777:83–92
Kudo K, Emmons GT, Casserly EW, Via DP, Smith LC, St. Pyrek J, Schroepfer GJ Jr (1989) Inhibitors of sterol synthesis. Chromatography of acetate derivatives of oxygenated sterols. J Lipid Res 30:1097–1111
Wasilchuk BA, Le Quesne PW, Vouros P (1992) Monitoring cholesterol autoxidation processes using multideuterated cholesterol. Anal Chem 64:1077–1087
Guardiola F, Codony R, Rafecas M, Boatella J (1995) Comparison of three methods for the determination of oxysterols in spray-dried egg. J Chromatogr A 705:289–304
Dyer RG, Hetherington CS, Alberti KGMM, Lake MF (1995) Simultaneous measurement of phytosterols (campesterol and beta-sitosterol) and 7-ketocholesterol in human lipoproteins by capillary column gas chromatography. J Chromatogr B 663:1–7
Dionisi FP, Golay A, Aeschlimann JM, Fay LB (1998) Determination of cholesterol oxidation products in milk powders: methods comparison and validation. J Agric Food Chem 46:2227–2233
Björkhem I, Breuer O, Angelin B, Wikstrom S (1988) Assay of unesterified cholesterol-5, 6-epoxide in human serum by isotope dilution mass spectrometry. Levels in the healthy state and in hyperlipoproteinemia. J Lipid Res 29:1031–1038
Breuer O, Bjorkhem I (1990) Simultaneous quantification of several cholesterol autoxidation and monohydroxylation products by isotope-dilution mass spectrometry. Steroid 55:185–192
Li S, Pang J, Wilson WK, Schroepfer GJ Jr (1999) Sterol synthesis. Preparation and characterization of fluorinated and deuterated analogs of oxygenated derivatives of cholesterol. Chem Phys Lipids 99:33–71
Shan H, Pang J, Li S, Chiang TB, Wilson WK, Schroepfer GJ Jr (2003) Chromatographic behavior of oxygenated derivatives of cholesterol. Steroids 68:221–233
Plat J, Brzezinka H, Lutjohann D, Mensink RP, von Bergmann K (2001) Oxidized plant sterols in human serum and lipid infusions as measured by combined gas-liquid chromatography-mass spectrometry. J Lipid Res 42:2030–2038
Grandgirard A, Martine L, Joffre CP, Juaneda P, Berdeaux O (2004) Gas chromatographic separation and mass spectrometric identification of mixtures of oxyphytosterol and oxycholesterol derivatives application to a phytosterol-enriched food. J Chromatogr A 1040:239–250
Bortolomeazzi R, Cordaro RF, Conte LS (2003) Presence of phytosterol oxides in crude vegetable oils and their fate during refining. J Agric Food Chem 51:2394–2401
Busch TJ, King AJ (2006) Artifact generation and monitoring in analysis of cholesterol oxide products. PhD thesis, University of California, Davis, CA
Guardiola F, Jordan A, Grau A, Garcia S, Boatella J, Rafecas M, Codony R (1998) Recent methodological advances in oxysterol determination. In: Pandalai SG (ed) Recent researched developments in oil chemistry. Transworld Research Network Kerala, India, pp 77–88
Grau A, Codony R, Grimpa S, Baucells MD, Guardiola F (2001) Cholesterol oxidation in frozen dark chicken meat: influence of dietary fat, source, alpha-tocopherol and ascorbic acid supplementation. Meat Sci 57:197–208
Guardiola F, Bou R, Boatella J, Codony R (2004) Analysis of sterol oxidation products in foods. J. AOAC Int 87:441–466
Louter AJ (2004) Determination of plant sterol oxidation products in plant sterol enriched spreads, fat blends, and plant sterol concentrates. J AOAC Int 485–492
van de Bovenkamp P, Kosmeijer-Schuil TG, Katan MB (1988) Quantification of oxysterols in Dutch foods: egg products and mixed diets. Lipids 23:1079–1085
Acknowledgments
We would like to acknowledge Heidrun B. Gross, University of California, Davis Department of Nutrition for her valuable advice, help, and support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Busch, T.P., King, A.J. Stability of Cholesterol, 7-Ketocholesterol and β-Sitosterol during Saponification: Ramifications for Artifact Monitoring of Sterol Oxide Products. J Am Oil Chem Soc 87, 955–962 (2010). https://doi.org/10.1007/s11746-010-1572-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-010-1572-3