Abstract
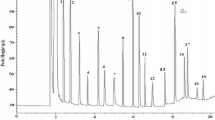
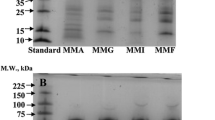
The unavailability of protein foods, particularly in the context of population growth, has been an important factor in the protein malnutrition encountered in developing countries. The fractionation, gel filtration and polyacrylamide gel electrophoresis (PAGE) of Ailanthus excelsa seed (a nontraditional source containing 15.81% protein) proteins were carried out in the present study, and their solubility profiles, surface topographies and amino acid compositions were evaluated. The globulin fraction dominated the seed protein composition, accounting for 51.31% (w/w) of the total soluble proteins in the seeds. Protein isolate and protein fractions of A. excelsa seeds showed similar topographical structures to those of other plant seed proteins. Analysis of the isolated proteins identified 17 amino acids, of which nine were essential. Gel filtration on Sephadex G-200 revealed the presence of seven components. PAGE detected different polypeptide bands in the range of 28.8−154.9 kDa in the protein isolate as well as in protein fractions for A. excelsa. The amino acid compositions, the solubility patterns and the high abundances of low molecular weight proteins indicate that the isolated seed protein of A. excelsa may be a potential food protein.



Similar content being viewed by others
References
Chopra RN, Nayar SL, Chopra IC (1956) Glossary of Indian medicinal plants. CSIR, New Delhi, p 10
Chatterjee A, Pakrashi SC (1995) Treatise on Indian medicinal plants, vol III. Publications and Information Directorate, CSIR, New Delhi, pp 60–61
Ogura M, Cordell GA, Kinghorn AD, Fransworth NR (1977) Potential anticancer agents VI. Constituents of Ailanthus excelsa. Lloydia 40:579–584
Joshi BC, Pandey A, Sharma RP, Khare A (2003) Quassinoids from Ailanthus excelsa. Phytochem 62:579–584
Shermann MM, Boris RP, Ogura M, Cordell GA, Fransworth NR (1980) 3S, 24S, 25-trihydroxytirucall-7-ene from A. excelsa. Phytochem 19:1499–1501
Shrimali M, Jain DC, Darokar MP, Sharma RP (2001) Antibacterial activity of Ailanthus excelsa. Phytother Res 15:165–166
Joshi BC, Pandey A, Churasia L, Pal M, Sharma RP, Khare A (2003) Antifungal activity of the stem bark of Ailanthus excelsa. Fitoterpia 74:689–691
Dhansekran S, Suresh B, Sethuraman M, Rajan S, Dubey R (1993) Antifertility activity of Ailanthus excelsa Roxb. in female albino rats. Indian J Exp Biol 31:384–385
Singh NP, Patnayak BC (1977) Nutritive value of Ailanthus excelsa Roxb. (Ardu) leaves for sheep. Indian Vet J 54(3):198–201
Nag A, Matai S (1994) Ailanthus excelsa Roxb. (Simaroubacae), a promising source of leaf protein. J Agric Food Chem 42:1115–1117
Guerra MJ, Park YK (1975) Extraction of sesame seed protein and determination of its molecular weight by sodium dodecyl sulphate–polyacrylamide gel electrophoresis. J Am Oil Chem Soc 52:73–84
Laskar S, Ghosh Majumder S, Basak B (1985) Isolation and chemical investigation of teak (Tectona grandis. Linn) seed protein. J Am Oil Chem Soc 62(8):1266–1268
Laskar S, Thakur S, Basak B (1988) Isolation, amino acid composition and molecular weight distribution of Eucalyptus kirtoniana seed proteins. J Am Oil Chem Soc 65(7):1131–1135
Basak B, Bhattacharya UK, Sinhababu A, Laskar S (1994) Extraction and chemical investigation of kulthi (Macrotylona uniflorus. Lam.) seed protein. App Biochem Biotech 49(3):281–290
Laskar S, Sinhababu A, Thakur S, Basak B (1998) Extraction and chemical investigation of Lagerstroemia speciosa seed proteins. J Am Lab 30(5):22–24
AOAC (1990) Official methods of analysis, 15th edn. Association of Official Analytical Chemists, Washington, DC
Osborne TB (1924) The vegetable proteins. Longmans, Green and Co., London, pp 51–56
Chavan UD, Mckenzie DB, Shahidi F (2001) Protein classification of beach pea (Lathyrus maritimus L.). Food Chem 75:145–153
Ghosh AK, Naskar AK, Sengupta S (1997) Characterization of a xylanolytic amyloglucosidase of Termitomyces clypeatus. Biochim Biophys Acta 1339:289–296
Whitaker JR (1963) Determination of molecular weights of proteins by gel filtration on Sephadex. Anal Chem 35:1950–1953
Leach AA, Óshea PC (1965) The determination of protein molecular weights of up to 225, 000 by gel filtration on a single column of Sephadex G-200 at 25° C and 40° C. J Chromatogr 17:245–251
Marcone MF (1999) Evidence confirming the existence of a 7S globulin like storage protein in Amaranthus hypochondriacus seed. Food Chem 65:533–548
Laemmli UK (1970) Cleavage of saturated proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Davis BJ (1964) Disc electrophoresis-II, method and application to human serum proteins. Ann N Y Acad Sci 121:404–427
Hazra KM, Laskar S (2005) Extraction and chemical investigation of Bakul (Mimusops elengi Linn.) seed protein. Adv Food Sci 27(3):116–120
Padilla FC, Alvarez MT, Alfero MJ (1996) Functional properties of barinas nut flour (Caryodendron orinocense Karst., Euphorbiaceae) compared to those of Soybean. Food Chem 57:191–196
Gilberg S, Toernell B (1976) Preparation of rapeseed protein isolate: precipitation of rapeseed proteins in presence of poly-acids. J Food Sci 41:1070–1075
Chan Chi-Wah, Phillips RD (1994) Amino acid composition and subunit constitution of protein fractions from cowpea (Vigna unguiculata L. Walp) seeds. J Agric Food Chem 42:1857–1860
Kahlon TS, Smith GE, Shao Q (2005) In vitro binding of bile acids by kidney bean (Phaseolus vulgaris), black gram (Vigna mungo), bengal gram (Cicer arietinum) and moth bean (Phaeolus aconitifolius). Food Chem 90:241–246
Subagio A (2006) Characterization of hyacinth bean (Lablab purpureus L. Sweet) seeds from Indonesia and their protein isolate. Food Chem 95:65–70
Sathe SK, Venkatachalam M (2007) Fractionation and biochemical characterization of moth bean (Vigna aconitifolia L.) proteins. LWT 40:600–610
Brul’e D, Savoie L (1998) In vitro digestibility of protein and amino acids in protein mixture. J Sci Food Agric 43:361–372
Acknowledgments
The authors are grateful to the DSA Project of the University Grants Commission, New Delhi, India, for financial assistance in this work.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kundu, P., Laskar, S. Fractionation and Some Chemical Studies on Ailanthus excelsa Roxb. Seed Protein. J Am Oil Chem Soc 85, 835–843 (2008). https://doi.org/10.1007/s11746-008-1273-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-008-1273-3