Abstract
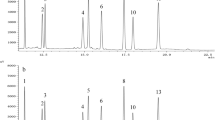
A new GC-MS method for characterization and quantification of phytosterol oxidation products was developed. Applicability of this method was tested by characterizing sitostanol oxides formed in bulk and then quantifying selected oxides in purified rapeseed oil and tripalmitin matrices in which the complex matrix made oxide analysis difficult. In bulk, nine different sitostanol oxides were characterized, including epimers of 7- and 15-hydroxysitostanol and 6- and 7-ketositostanol. In both lipid matrices, the amounts of sitostanol oxides generated in thermo-oxidation were very low. According to statistical analyses, depending on the oxide, the GC-MS results were the same or slightly higher than those quantified by the more common GC-FID method. Thus, GC-MS provides a powerful alternative for characterization and quantification of phytostanol oxides found in low amounts in complex matrices and is a promising method for future phytosterol oxide studies.
Similar content being viewed by others
References
Moreau, R.A., B.D. Whitaker, and K.B. Hicks, Phytosterols, Phytostanols, and Their Conjugates in Foods: Structural Diversity, Quantitative Analysis, and Health-Promoting Uses, Prog. Lipid Res. 41:457–500 (2002).
Jones, P.J., and M. Raeini-Sarjaz, Plant Sterols and Their Derivatives: The Current Spread of Results, Nutr. Rev. 59:21–24 (2000).
Ratnayake, W.M.N., and E. Vavasour, Potential Health Risks Associated with Large Intakes of Plant Sterols, in Plant Sterols: Analytical, Nutritional, and Safety Aspects as Functional Food Components, edited by P.C. Dutta, Marcell Dekker, New York, 2003, pp. 365–395.
Dutta, P.C., and G.P. Savage, Formation and Content of Phytosterol Oxidation Products in Foods, in Cholesterol and Phytosterol Oxidation Products: Analysis, Occurrence, and Biological Effects, edited by F. Guardiola, P.C. Dutta, R. Codony, and G.P. Savage, AOCS Press, Champaign, 2002, pp. 319–334.
Grandgirard, A., J.-P. Sergiel, M. Nour, J. Demaison-Meloche, and C. Giniès, Lymphatic Absorption of Phytosterol Oxides in Rats, Lipids 34:563–570 (1999).
Meyer, W., H. Jungnickel, M. Jandke, K. Dettner, and G. Spiteller, On the Cytotoxity of Oxidized Phytosterols Isolated from Photoautotrophic Cell Cultures of Chenopodium rubrum Tested on Meal-Worms Tenebrio molitor, Phytochemistry 47: 789–797 (1998).
Adcox, C., L. Boyd, L. Oerhl, J. Allen, and G. Fenner, Comparative Effects of Phytosterol Oxides and Cholesterol Oxides in Cultured Macrophage-derived Cell Lines, J. Agric. Food Chem. 49:2090–2095 (2001).
Nourooz-Zadeh, J., and L.-Å. Appelqvist, Isolation and Quantitative Determination of Sterol Oxides in Plant-based Foods: Soybean Oil and Wheat Flour, J. Am. Oil Chem. Soc. 69:288–293 (1992).
Dutta, P.C., and L.-Å. Appelqvist, Studies on Phytosterol Oxides. I: Effect of Storage on the Content in Potato Chips Prepared in Different Vegetable Oils, 74:647–657 (1997).
Dutta, P.C., Studies on Phytosterol Oxides. II: Content in Some Vegetable Oils and in French Fries Prepared in These Oils, 74:659–666 (1997).
Dutta, P.C., R. Przybylski, L.-Å. Appelqvist, and N.A.M. Eskin, Formation and Analysis of Oxidized Sterols in Frying Fat, in Deep Frying: Chemistry, Nutrition, and Practical Applications, edited by E.G. Perkins and M.D. Erickson, AOCS Press, Campaign, 1996, pp. 112–150.
Dutta, P.C., Determination of Phytosterol Oxidation Products in Foods and Biological Samples, in Cholesterol and Phytosterol Oxidation Products: Analysis, Occurrence, and Biological Effects, edited by F. Guardiola, P.C. Dutta, R. Codony, and G.P. Savage, AOCS Press, Campaign, 2002, pp. 335–374.
Lampi, A.-M., L. Juntunen, J. Toivo, and V. Piironen, Determination of Thermo-oxidation Products of Plant Sterols, J. Chromatogr. B 777:83–92 (2002).
Guardiola, F., J. Boatella, and R. Codony, Determination of Cholesterol Oxidation Products by Gas Chromatography, in Cholesterol and Phytosterol Oxidation Products: Analysis, Occurrence, and Biological Effects, edited by F. Guardiola, P.C. Dutta, R. Codony, and G.P. Savage, AOCS Press, Campaign, 2002, pp. 50–65.
Blekas, G., and D. Boskou, Oxidation of Stigmasterol in Heated Triacylglycerols, Food Chem. 33:301–310 (1989).
Lampi, A.-M., L.H. Dimberg, and A. Kamal-Eldin, A Study on the Influence of Fucosterol on Thermal Polymerisation of Purified High Oleic Sunflower Triacylglycerols, J. Sci. Food Agric. 79:573–579 (1999).
Kamal-Eldin, A., S. Görgen, J. Petterson, and A.-M. Lampi, Normal-Phase High-Performance Liquid Chromatography of Tocopherols and Tocotrienols. Comparison of Different Chromatographic Columns, J. Chromatogr. A 881:217–227 (2000).
Aringer, L., and L. Nordström, Chromatographic Properties and Mass Spectrometric Fragmentation of Dioxygenated C27-, C28-, C29-Steroids, Biomed. Mass Spectrom. 8:183–203 (1981).
Säynäjoki, S., S. Sundberg, L. Soupas, A.-M. Lampi, and V. Piironen, Determination of Stigmasterol Primary Oxidation Products by High-Performance Liquid Chromatography, Food Chem. 80:415–421 (2003).
Brooks, C.J.W., E.C. Horning, and J.S. Young, Characterization of Sterols by Gas Chromatography-Mass Spectrometry of the Trimethylsilyl Ethers, Lipids 3:391–402 (1968).
Bortolomeazzi, R., M. De Zan, L. Pizzale, and L.S. Conte, Mass Spectrometry Characterization of the 5α-, 7α-, and 7β-Hydroxy Derivatives of β-Sitosterol, Campesterol, Stigmasterol, and Brassicasterol, J. Agric. Food Chem. 47:3069–3074 (1999).
Lebovics, V.K., M. Antal, and Ö. Gaàl, Enzymatic Determination of Cholesterol Oxides, J. Sci. Food Agric. 71:22–26 (1996).
Horwitz, W., International Coordination and Validation of Analytical Methods, Food Addit. Contam. 10:61–69 (1993).
Dutta, P.C., and G.P. Savage, Harmonization of Cholesterol Oxidation Product Analysis, in Cholesterol and Phytosterol Oxidation Products: Analysis, Occurrence, and Biological Effects, edited by F. Guardiola, P.C. Dutta, R. Codony, and G.P. Savage, AOCS Press, Champaign, 2002, pp. 114–123.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Soupas, L., Juntunen, L., Säynäjoki, S. et al. GC-MS method for characterization and quantification of sitostanol oxidation products. J Amer Oil Chem Soc 81, 135–141 (2004). https://doi.org/10.1007/s11746-004-0871-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-004-0871-1