Abstract
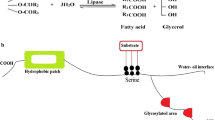
The lipase-catalyzed esterification of sorbitan with oleic acid in a solvent-free system to form sorbitan oleate (commercial name Span80) was studied as a feasible approach aimed at meeting the demand for sugar alcohol-based surfactants. Screened results obtained from enzymatic synthesis of sorbitan oleate indicated that Novozym 435 had its highest catalytic activity in a solvent-free system. The introduction of a reduced-pressure system increased the production of sorbitan oleate to a maximum of 95% of theoretical, obtained from 0.2 mol sorbitan, 0.1 mol oleic acid, and 2.0 g lipase (6 wt% of sorbitan) in a solvent-free reaction mixture at optimal reaction conditions. Results obtained from lipase-catalyzed batch esterification reactions showed that more than 90% conversion of sorbitan oleate was maintained after 10 batches of esterification reactions, indicating excellent enzyme stability. Subsequent analysis by HPLC indicated that the product of enzyme-catalyzed esterification by the immobilized lipase contained a significantly greater amount of monoester (about 80%) compared to the composition obtained by chemical synthesis (about 50%).
Similar content being viewed by others
References
Sarney, D.B., M.J. Barnard, M. Virto, and E.N. Vulfson, Enzymatic Synthesis of Sorbitan Esters Using a Low-Boiling-Point Azeotrope as a Reaction Solvent, Biotechnol. Bioeng. 54:351–356 (1997).
Ducret, A., A. Giroux, M. Trani, A. Ducret, A. Giroux, M. Trani, and R. Lortie, Enzymatic Preparation of Biosurfactants from Sugars or Sugar Alcohols and Fatty Acids in Organic Media Under Reduced Pressure, Ibid. 8:214–221 (1995).
Jose, A.A., M. Bernabe, and C. Otero, Quantitative Enzymatic Production of 1.6-Diacyl Sorbitol Esters, Ibid. 60:53–60 (1998).
Sarney, D.B., and E.N. Vulfson, Application of Enzyme to the Synthesis of Surfactants, Trends Biotechnol. 13:164–172 (1995).
Pathak, T., and H. Waldmann Enzymatic Protecting Group Techniques in Organic Synthesis, in Stereoselective Biocatalysis, edited by R.N. Patel, Marcel Dekker, New York, 2000, pp. 775–797.
Jansen, A.E.M., A.G. Lefferts, and K. Van't Riet, Enzymatic Synthesis of Carbohydrate Esters in Aqueous Media, Biotechnol. Lett. 12:711–716 (1990).
Ducret, A., A. Giroux, M. Trani, and R. Lortie, Characterization of Enzymatically Prepared Biosurfactants, J. Am. Oil Chem. Soc. 73:109–113 (1996).
Fregapane, G., D.B. Sarney, S.G. Greenberg, D.J. Knight, and E.N. Vulfson, Enzymatic Synthesis of Monosaccharide Fatty Acid Esters and Their Comparison with Conventional Products, Ibid. 71:87–91 (1994).
Vulfson, E.N., Enzymatic-Synthesis of Food Ingredients in Low-Water Media, Trends Food Sci. Technol. 4:209–215 (1993).
Grati, N., E. Wellner, A. Asrin, and S. Sarig, Analysis of Sorbitan Fatty Acid Esters by HPLC, J. Am. Oil Chem. Soc. 60: 1151–1154 (1983).
Björkling, F., S.E. Godtfredsen, and O. Kirk, The Future Impact of Industrial Lipases, Trends Biotechnol. 9:360–363 (1991).
Cao, L., A. Fischer, U.T. Bornscheuer, and R.D. Schmid, Lipase-Catalyzed Solid Phase Synthesis of Sugar Fatty Acid Esters, Biocatal. Biotransform. 14:269–283 (1997).
Mukesh, D., D. Sheth, A. Mokashi, J. Wagh, J.M. Tilak, A.A. Banerji, and K.R. Thakkar, Lipase Catalysed Esterification of Isosorbide and Sorbitol, Biotechnol. Lett. 15:1243–1246 (1993).
Chopineau, J., F.D. McCafferty, M. Therisod, and A.M. Klibanov, Production of Biosurfactants from Sugar Alcohols and Vegetable Oils Catalyzed by Lipases in Nonaqueous Medium, Biotechnol. Bioeng. 31:208–214 (1988).
Xu, Y., D. Wang, X.Q. Mu, G.A. Zhao, and K.C. Zhang, Biosynthesis of Ethyl Esters of Short-Chain Fatty Acids Using Whole-Cell Lipase from Rhizopus chinensis CCTCC M201021 in Non-aqueous Phase, J. Mol. Catal. B: Enzymatic 18:29–37 (2002).
Stockburger, G.J., Process for Preparing Sorbitan Esters, U.S. Patent 4,297,290 (1981).
Van der Padt, A., J.J.W. Sewalt, and K. Van't Riet, On-line Water Removal During Enzymatic Triacylglycerol Synthesis by Means of Pervaporation, J. Membrane Sci. 80:199–208 (1993).
Yan, Y., U.T. Bornscheuer, and R.D. Schmid, Efficient Water Removal in Lipase-Catalyzed Esterifications Using a Low-Boiling-Point Azeotrope, Biotechnol. Bioeng. 78:31–34 (2002).
Guillardeau, L., D. Montet, N. Khaled, M. Pina, and J. Graille, Fructose Caprylate Biosynthesis in a Solvent-Free Medium, Tenside Surfact. Deterg. 25:342–344 (1992).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Xu, Y., Wang, D., Mu, X.Q. et al. Efficient esterification of sorbitan oleate by lipase in a solvent-free system. J Amer Oil Chem Soc 80, 647–651 (2003). https://doi.org/10.1007/s11746-003-0753-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11746-003-0753-6