Abstract
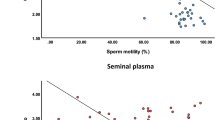
Docosahexaenoic acid (DHA; 22∶6n−3) is found in extremely high levels in human ejaculate with the majority occurring in the spermatozoa. However, the relative concentration of DHA and other fatty acids, in blood serum, seminal plasma, and spermatozoa of asthenozoospermic vs. normozoospermic individuals is not known. We analyzed the phospholipid fatty acid composition of blood serum, seminal plasma, and spermatozoa of normozoospermic men and asthenozoospermic men in order to determine if DHA levels, as well as the levels of other fatty acids, differed. The serum phospholipid DHA levels were similar in the two groups, suggesting similar intakes of dietary DHA. On the other hand, seminal plasma levels of DHA (3.0 vs. 3.7%) and total polyunsaturated fatty acids (PUFA) (11.8 vs. 13.5%) were significantly lower in asthenozoospermic vs. normozoospermic men, respectively, while 18∶1 (19.0 vs. 16.8%) and monounsaturated fatty acids (MUFA) (24.2 vs. 21.7%) were significantly higher in the asthenozoospermic vs. the normozoospermic men. Spermatozoa from asthenozoospermic men had higher levels of 18∶1, 20∶0, 22∶0, 22∶1, and 24∶0 than sperm from normozoospermic men, and lower levels of 18∶0 and DHA (8.2 vs. 13.8%). Furthermore, total MUFA (19.3 vs. 16.5%) was higher and total PUFA (19.0 vs. 24.0%), n−3 fatty acids (9.3 vs. 14.6%), and the ratio of n−3 to n−6 fatty acids (1.0 vs. 1.6) were lower in the asthenozoospermic men. Therefore, in asthenozoospermic individuals, lower levels of DHA in the seminal plasma, but not in the blood serum, mimic the decreased concentrations of DHA in the spermatozoa. This suggests that the lower concentrations of spermatozoon DHA in these individuals are due not to dietary differences but to some type of metabolic difference in the asthenozoopermic men.
Similar content being viewed by others
Abbreviations
- DHA:
-
docosahexaenoic acid
- EPA:
-
eicosapentaenoic acid
- MUFA:
-
monounsaturated fatty acid
- PL:
-
phospholipid
- PUFA:
-
polyunsaturated fatty acid
References
Cates, W., Farley, T.M.M., and Rowe, P.J. (1985) World Wide Patterns of Infertility: Is Africa Different, Lancet 1, 596–598.
Baker, H.G.W., Gurger, H.G., de Kretser, D.M., and Hudson, B. (1986) Relative Incidence of Etiologic Disorders in Male Infertility, in Male Reproductive Dysfunction (Sauten, R.S. and Swerdloff, R.S., eds.) pp. 341–372 Marcel Dekker, New York.
Cai, X., and Marik, J.J. (1989) Improving Penetrating Capacity of Spermatozoa with Poor Motility by Addition of Caffeine at Coincubation with Zona Free Hamster Ova, Fertil. Steril. 51, 719–721.
Nissen, H.P., and Kreysel, H.W. (1983) Polyunsaturated Fatty Acids in Relation to Sperm Motility, Andrologia 15, 264–269.
Gulaya, N.M., Tronko, M.D., Volkov, G.L., and Margitich, M. (1993) Lipid Composition and Fertile Ability of Human Ejaculate, Ukr. J. Biochem. 65, 64–70.
Cerolini, S., Kelso, K.A., Noble, R.C., Speake, B.K., Pizzi, F., and Cavalchini, L.G. (1997) Relationship Between Spermatozoan Lipid Composition and Fertility During Aging of Chickens, Biol. Reprod. 57, 976–980.
Zalata, A.A., Christophe, A.B., Depuydt, C.E., Schoonjans, F., and Comhaire, F.H. (1998) The Fatty Acid Composition of Phospholipids of Spermatozoa from Infertile Patients, Mol. Hum. Reprod. 4, 111–118.
Connor, W.E., Weleber, R.G., DeFrancesco, C., Lin, D.S., and Wolf, D.P. (1997) Sperm Abnormalities in Retinitis Pigmentosa, Invest. Opthalmol. Visual Sci. 38, 2619–2628.
Zalata, A.A., Christophe, A.B., Depuydt, C.E., Schoonjans, F., and Comhaire, F.H. (1998) White Blood Cells Cause Oxidative Damage to the Fatty Acid Composition of Phospholipids of Human Spermatozoa, Int. J. Androl. 21, 154–162.
Lin, D.S., Connor, W.E., Wolf, D.P., Neuringer, M., and Hachey, D.L. (1993) Unique Lipids of Primate Spermatozoa: Desmosterol and Docosahexaenoic Acid, J. Lipid Res. 34, 491–499.
Connor, W.E., Lin, D.S., Wolf, D.P., and Alexander, M. (1998) Uneven Distribution of Desmosterol and Docosahexaenoic Acid in the Heads and Tails of Monkey Sperm, J. Lipid Res. 39, 1404–1411.
Jones, R.E., and Plymate, S.R. (1993) Synthesis of Docosahexaenoyl Coenzyme A in Human Spermatozoa, J. Androl. 14, 428–432.
Jones, R.E., and Plymate, S.R. (1988) Evidence for the Regulation of Fatty Acid Utilization in Human Sperm by Docosahexaenoic Acid, Biol. Reprod. 39, 76–80.
Oliw, E.H., and Sprecher, H.W. (1991) Metabolism of Polyunsaturated (n−3) Fatty Acids by Monkey Seminal Vesicles: Isolation and Biosynthesis of Omega-3 Epoxides, Biochim. Biophys. Acta 1086, 287–294.
Ma, J., Folsom, A.R., Eckfeldt, J.H., Lewis, S.L., and Chambless, L.E. (1995) Short-and Long-Term Repeatability of Fatty Acid Composition of Human Plasma Phospholipids and Cholesterol Esters, Am. J. Clin. Nutr. 62, 572–578.
Bonaa, K.H., Bjerve, K.S., and Nordoy, A. (1992) Habitual Fish Consumption, Plasma Phospholipid Fatty Acids, and Serum Lipids: The Tromso Study, Am. J. Clin. Nutr. 55, 1126–1134.
Nikkari, T., Luukkainen, P., Pietinen, P., and Buska, P. (1995) Fatty Acid Composition of Serum Lipid Fractions in Relation to Gender and Quality of Dietary Fat, Ann. Med. 27, 491–498.
Mortimer, D. (1985) The Male Factor in Infertility Part II. Sperm Function Testing, in Curr. Probl. Obstet. Gynecol. Fertil., Vol. 8, p. 75, Year Book Medical Publishers, Chicago.
World Health Organization (1992) WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interactions, Cambridge University Press, Cambridge.
Folch, J., Lees, M., and Sloane Stanley, G.H. (1957) A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues, J. Biol. Chem. 226, 497–509.
Bligh, E.G., and Dyer, W.I. (1959) A Rapid Method of Total Lipid Extraction and Purification, Can. J. Biochem. Physiol. 37, 911–917.
Conquer, J.A., and Holub, B.J. (1996) Supplementation with an Algae Source of Docosahexaenoic Acid Increases (n−3) Fatty Acid Status and Alters Selected Risk Factors for Heart Disease in Vegetarian Subjects, J. Nutr. 126, 3032–3039.
Conquer, J.A., and Holub, B.J. (1997) Dietary Docosahexaenoic Acid as a Source of Eicosapentaenoic Acid in Vegetarians and Omnivores, Lipids 32, 341–345.
Conquer, J.A., and Holub, B.J. (1998) Effect of Supplementation with DHA on Lipid/Lipoprotein Levels in Subjects of Asian Indian Background, J. Lipid Res. 39, 286–292.
Emken, E.A., Adlof, R.O., and Gulley, R.M. (1994) Dietary Linoleic Acid Influences Desaturation and Acylation of Deuterium-Labelled Linoleic Acid and Linolenic Acids in Young Adult Males, Biochim. Biophys. Acta 1213, 277–288.
Lenzi, A., Picardo, M., Gandini, L., Lombardo, F., Terminali, O., Passi, S., and Dondero, F. (1994) Glutathione Treatment of Dyspermia: Effect on the Lipoperoxidation Process, Hum. Reprod. 9, 2044–2050.
Vignon, F., Koll-Back, M.H., Clavert, A., and Cranz, C. (1989) Lipid Composition of Human Seminal Plasma, Arch. Androl. 22, 49–53.
Aitken, R.J. (1995) Free Radicals, Lipid Peroxidation and Sperm Function, Reprod. Fertil. Dev. 7, 659–668.
Mitropoulos, D., Deliconstantinos, G., Zervas, A., Villiotou, V., Dimopoulos, C., and Stavrides, J. (1996) Nitric Oxide Synthase and Xanthine Oxidase Activities in the Spermatic Vein of Patients with Varicocele: A Potential Role for Nitric Oxide and Peroxynitrite in Sperm Dysfunction, J. Urol. 156, 1952–1958.
Alkan, I., Simsek, F., Goncagul, H., Kervancioglu, E., Ozveri, H., Yalcin, S., and Akdas, A. (1997) Reactive Oxygen Species Production by the Spermatozoa of Patients with Idiopathic Infertility: Relationship to Seminal Plasma Antioxidants, J. Urol. 157, 140–143.
Lewis, S.E.M., Sterling, E.S.L., Young, I.S., and Thompson, W. (1997) Comparison of Individual Antioxidants of Sperm and Seminal Plasma in Fertile and Infertile Men, Fertil. Steril. 67, 142–147.
Christophe, A., Zalata, A., Mahmoud, A. and Combaire, F. (1998) Fatty Acid Composition of Sperm Phospholipids and Its Nutritional Implications, Middle East Fertil. Soc. J. 3, 46–53.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Conquer, J.A., Martin, J.B., Tummon, I. et al. Fatty acid analysis of blood serum, seminal plasma, and spermatozoa of normozoospermic vs. Asthernozoospermic males. Lipids 34, 793–799 (1999). https://doi.org/10.1007/s11745-999-0425-1
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-999-0425-1