Abstract
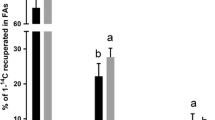
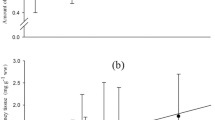
The aim of this study was to measure the changes in lipid metabolism which occur during smoltification and seawater transfer in Atlantic salmon (Salmo salar). Duplicate groups of Atlantic salmon parr were fed diets containing either fish oil (FO) or a blend of linseed and rapeseed oils, vegetable oil (VO), from October (week 0) to seawater transfer in May (week 26). From May to August (weeks 26–43), all fish were fed a fish oil-containing diet. Fatty acyl desaturation and elongation activity were followed in isolated hepatocytes incubated with radioactive 18:3n−3 and 18:2n−6. Metabolism of 18:3n−3 was consistently around 5-fold greater than metabolism of 18:2n−6, and total metabolism of both substrate polyunsaturated fatty acids (PUFA) was increased in fish fed both VO and FO up to seawater transfer after which desaturation activities were reduced. Desaturation activities with both 18:3n−3 and 18:2n−6 were significantly greater in fish fed VO, compared to fish fed FO, at 22 and 26 wk. Arachidonic acid (20:4n−6; AA) in liver polar lipids (PL) of fish fed VO increased consistently from weeks 0–22 but varied after seawater transfer. In fish fed FO, AA in liver PL remained constant up to week 17 before increasing at seawater transfer and leveling off thereafter. Eicosapentaenoic acid (20:5n−3; EPA) in liver PL of fish fed VO decreased significantly from week 0–22 before rising at seawater transfer and increasing rapidly posttransfer. EPA in liver PL of fish fed FO showed a similar trend except EPA was always greater in the freshwater phase compared to fish fed VO. Docosahexaenoic acid (DHA) levels in liver PL of fish fed VO remained constant in the freshwater phase before increasing following seawater transfer. In fish fed FO, DHA in liver PL increased from weeks 0–17 reducing and leveling off postseawater transfer. The levels of PGF2α and PGF3α were measured in isolated gill cells stimulated with calcium ionophore A23187. PGF2α production in fish fed VO increased significantly between 0–7 wk before decreasing toward seawater transfer. After transfer, PGF2α production increased to a peak at 35 wk. PGF2α production in fish fed FO was not significantly altered during the trial period. The changes in PGF3α production were broadly similar to those occurring with PGF2α, but the latter was always in excess of the former (2-to 4-fold). Plasma chloride concentrations in fish subjected to seawater challenge at 20 wk were significantly lower in fish fed VO compared to those fed FO. This study has provided new information on the changes in lipid metabolism which accompany parr-smolt transformation and suggests that diets which have a fatty acid composition more similar to that in aquatic invertebrates may be beneficial in effecting successful seawater adaptation.
Similar content being viewed by others
Abbreviations
- AA:
-
arachidonic acid
- BHT:
-
butylated hydroxytoluene
- DHA:
-
docosahexaenoic acid
- EFA:
-
essential fatty acid
- EPA:
-
eicosapentaenoic acid
- FAF-BSA:
-
fatty acid free bovine serum albumin
- FO:
-
fish oil
- HBSS:
-
Hank’s balanced salt solution
- MHM:
-
Marine Harvest McConnell Ltd.
- PGF:
-
prosta-glandin F
- PL:
-
polar lipid
- PUFA:
-
polyunsaturated fatty acid
- TLC:
-
thin-layer chromatography
- VO:
-
vegetable oil
References
Bell, J.G., Dick J.R., and Sargent, J.R. (1993) Effect of Diets Rich in Linoleic or α-Linolenic Acid on Phospholipid Fatty Acid Composition and Eicosanoid Production in Atlantic Salmon (Salmo salar), Lipids 28, 819–826.
Bell, J.G., Tocher, D.R., MacDonald, F.M., and Sargent, J.R. (1995) Effects of Dietary Borage Oil [Enriched in γ-Linolenic Acid, 18:3(n−6)] on Growth, Mortalities, Liver Histopathology, and Lipid Composition of Juvenile Turbot (Scophthalmus maximus), Fish Physiol. Biochem. 14, 373–383.
Henderson, R.J., and Tocher, D.R. (1987) The Lipid Composition and Biochemistry of Freshwater Fish, Prog. Lipid Res. 26, 281–347.
Sargent, J.R., Henderson, R.J., and Tocher, D.R. (1989) The Lipids, in Fish Nutrition (Halver, J.E., ed.) Academic Press, New York, pp. 154–218.
Sargent, J.R., Bell, M.V., Bell, J.G., Henderson, R.J., and Tocher, D.R. (1993) Origins and Functions of n−3 Polyunsaturated Fatty Acids in Marine Organisms, in Phospholipids: Characterization, Metabolism and Novel Biological Applications (Ceve, G., and Paltauf, F., eds.) AOCS Press, Champaign.
Hanson, B.J., Cummins, K.W., Cargill, A.S., and Lowry, R.R. (1985) Lipid Content, Fatty Acid Composition, and the Effect of Diet on Fats of Aquatic Insects, Comp. Biochem. Physiol., 80B, 257–276.
Bell, J.G., Ghioni, C., and Sargent, J.R. (1994) Fatty Acid Compositions of 10 Freshwater Invertebrates Which Are Natural Food Organisms of Atlantic Salmon (Salmo salar): A Comparison with Commercial Diets, Aquaculture 128, 301–313.
Folmar, L.C., and Dickhoff, W.W. (1980) The Parr-Smolt Transformation (Smoltification) and Seawater Adaptation in Salmonids: A Review of Selected Literature, Aquaculture 21, 1–37.
Hoar, W.S. (1976) Smolt Transformation: Evolution, Behaviour and Physiology, J. Fish Res. Board Can., 33, 1234–1252.
Wedermeyer, G.A., Saunders, R.L., and Clarke, W.C. (1980) Environmental Factors Affecting Smoltification and Early Marine Survival of Anadromous Salmonids, Mar. Fish. Rev. 42, 1–14.
Sheridan, M.A., Allen, W.V., and Kerstetter, T.H. (1985) Changes in the Fatty Acid Composition of Steelhead Trout, Salmo gairdnerii Richardson Associated with Parr-Smolt Transformation, Comp. Biochem. Physiol. 80B, 671–676.
Li, H.-O., and Yamada, J. (1992) Changes of the Fatty Acid Composition in Smolts of Masu Salmon (Oncoryhnchus masou), Associated with Desmoltification and Seawater Transfer, Comp. Biochem. Physiol. 103A, 221–226.
Ackman, R.G., and Takeuchi, T. (1986) Comparison of Fatty Acids and Lipids of Smolting Hatchery-Fed and Wild Atlantic Salmon Salmo salar, Lipids 21, 117–120.
Bolgova, O.M., and Shchurov, I.L. (1987) Adaptive Changes in Fatty Acid Spectra in Tissue Lipids of Wild and Cultivated Salmon Salmo salar During the Parr-Smolt Period, Zh. Evol. Biokhim. Fiziol, 23, 211–215.
Bell, J.G. (1996) Influences of Dietary Polyunsaturated Fatty Acid Composition and Eicosanoid Production in Atlantic Salmon (Salmo salar), Thesis submitted for degree of Doctor of Philosophy, University of Stirling, Scotland, United Kingdom.
Di Constanzo, G., Duportail, G., Florents, A., and Leray, C. (1983) The Brush Border Membrane of Trout Intestine: Influence of Its Lipid Composition on Ion Permeability, Enzyme Activity and Membrane Fluidity, Mol. Physiol. 4, 279–290.
Bell, M.V., Henderson, R.J., Pirie, B.J.S., and Sargent, J.R. (1985) Effects of Dietary Polyunsaturated Fatty Acid Deficiencies of Mortality and Gill Structure in the Turbot Scophthalmus maximus, J. Fish. Biol. 26, 181–191.
Spector, A.A., and Yorek, M.A. (1985) Membrane Lipid Composition and Cellular Function, J. Lipid Res. 26, 1015–1035.
Gerbi, A., Zerouga, M., Debray, M., Durand, G., Chanez, C., and Bourre, J.-M. (1994) Effect of Fish Oil Diet on Fatty Acid Composition of Phospholipids of Brain Membranes and on Kinetic Properties of Na+, K+-ATPase Isoenzymes of Weaned and Adult Rats, J. Neurochem. 62, 1560–1569.
Tocher, D.R., and Sargent, J.R. (1987) The Effects of Calcium Ionophore A23187 on the Metabolism of Arachidonic and Eicosapentaenoic Acids in Neutrophils from a Marine Teleost Fish Rich in (n−3) Polyunsaturated Fatty Acids, Comp. Biochem. Physiol. 87B, 733–739.
Bell, J.G., Tocher, D.R., and Sargent, J.R. (1994) Effect of Supplementation with 20:3(n−6), 20:4(n−6) and 20:5n−3 on the Production of Prostaglandins E and F of the 1-,2- and 3-Series in Turbot (Scophthalmus maximus) Brain Astroglial Cells in Primary Culture, Biochim. Biophys. Acta 1211, 335–342.
Brown, J.A., and Bucknall, R.M. (1986) Antidiuretic and Cardiovascular Actions of PGE2 in the Rainbow Trout Salmo gairdneri, Gen. Comp. Endocrinol. 61, 330–337.
Mustafa, T., and Srivastava, K.C. (1989) Prostaglandins (Eicosanoids) and Their Role in Ectothermic Organisms, Adv. Comp. Environ. Physiol. 5, 157–207.
U.S. National Research Council (1993) Nutrient Requirements of Fish, 114 pp., National Academy Press, Washington D.C.
Folch, J., Lees, M., and Sloane-Stanley, G.H. (1957) A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissues, J. Biol. Chem. 226, 497–509.
Christie, W.W. (1982) Lipid Analysis, 2nd edn., Pergamon Press, Oxford, p. 207.
Ackman, R.G. (1980) Fish Lipids Part 1, in Advances in Fish Science and Technology, (Connell, J.J., ed.) pp. 87–103, Fishing News Books, Farnham, United Kingdom.
Tocher, D.R., Sargent, J.R., and Frerichs, G.N. (1988) The Fatty Acid Compositions of Established Fish Cell Lines After Long-Term Culture in Mammalian Sera, Fish Physiol. Biochem. 5, 219–227.
Wilson, R., and Sargent, J.R. (1992) High-Resolution Separation of Polyunsaturated Fatty Acids by Argentation Thin-Layer Chromatography, J. Chromatogr. 623, 403–407.
Powell, W.S. (1982) Rapid Extraction of Arachidonic Acid Metabolites from Biological Samples Using Octadecyl Silica, Methods Enzymol. 86, 467–477.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J. (1951) Protein Measurement with the Folin Phenol Reagent, J. Biol Chem. 193, 265–275.
Sprecher, H. (1989) (n−3) and (n−6) Fatty Acid Metabolism, in Dietary Omega 3 and Omega 6 Fatty Acids. Biological Effects and Nutritional Essentiality (Galli, C., and Simopoulos, A.P., eds.) pp. 69–79, NATO ASI series. Series A: Life Sciences, Vol. 171, Plenum Press, New York and London.
Yu, T.C., and Sinnhuber, R.O. (1979) Effect of Dietary ω3 and ω6 Fatty Acids on Growth and Feed Conversion Efficiency of Coho Salmon (Oncorhynchus kisutch), Aquaculture 16, 31–38.
Tocher, D.R., and Dick, J.R. (1990) Polyunsaturated Fatty Acid Metabolism in Cultured Fish Cells: Incorporation and Metabolism of (n−3) and (n−6) Series Acids by Atlantic Salmon (Salmo salar) Cells, Fish Physiol. Biochem. 8, 311–319.
Garg, M.L., Sebokova, E., Thomson, A.B.R., and Clandinin, M.T. (1988) Δ6-Desaturase Activity in Liver Microsomes of Rats Fed Diets Enriched with Cholesterol and/or n−3 Fatty Acids, Biochem. J. 249, 351–356.
Garg, M.L., Thomson, A.B.R., and Clandinin, M.T. (1988) Effect of Dietary Cholesterol and/or n−3 Fatty Acids on Lipid Composition and Δ5-Desaturase Activity of Rat Liver Microsomes, J. Nutr. 118, 661–668.
Leger, C., Fremont, L., and Boudon, M. (1981) Fatty Acid Composition of Lipids in the Trout-1. Influence of Dietary Fatty Acids on the Triglyceride Fatty Acid Desaturation in Serum, Adipose Tissue, Liver, White, and Red Muscle, Comp. Biochem. Physiol. 69B, 99–105.
Ackman, R.G. (1967) Characteristics of the Fatty Acid Composition and Biochemistry of Some Freshwater Fish Oils and Lipids in Comparison with Marine Oils and Lipids, Comp. Biochem. Physiol. 22, 907–922.
Gong, B., and Farrell, A.P. (1990) Comparison of Blood and Muscle Levels of Unsaturated Fatty Acids in Parr and Mature Coho Salmon, Comp. Biochem. Physiol. 96B, 483–486.
Hazel, J.R. (1990) Adaptation to Temperature: Phospholipid Synthesis in Hepatocytes of Rainbow Trout, Am. J. Physiol. 258, R1495–1501.
Leray, C., Chapelle, S., Duportail, G., and Lorenz, A.F. (1984) Changes in Fluidity and 22:6(n−3) Content in Phospholipids of Trout Intestinal Brush-Border Membrane as Related to Environmental Salinity, Biochim. Biophys. Acta 778, 233–238.
Daikoku, T., Yano, I., and Masui, M. (1982) Lipid and Fatty Acid Compositions and Their Changes in the Different Organs and Tissues of Guppy, Poecilia reticulata, on Seawater Adaptation, Comp. Biochem. Physiol. 73A, 167–174.
Salem, N., Shingu, T., Kim, H.Y., Hullin, F., Bougnoux, P., and Karanian, J.W. (1988) Abberrations in Membrane Structure and Function, in Biological Membranes (Karnovsky, M.L., Bolis, L., and Leaf, A., eds.) pp. 319–333, Alan R. Liss, New York.
Anderson, A.A., Fletcher, T.C., and Smith, G.M. (1981) Prostaglandin Biosynthesis in the Skin of Plaice, Pleuronectes platessa L., Comp. Biochem. Physiol. 70C, 195–199.
Nalbone, G., Grynberg, A., Chevalier, A., Leonardi, J., Termine, E., and Lafont, H. (1990) Phospholipase A Activity of Cultured Rat Ventricular Myocyte Is Affected by the Nature of Cellular Polyunsaturated Fatty Acids, Lipids 25, 301–306.
Bell, J.G., Dick, J.R., McVicar, A.H., Sargent, J.R., and Thompson, K.D. (1993) Dietary Sunflower, Linseed and Fish Oils Affect Phospholipid Fatty Acid Composition, Development of Cardiac Lesions, Phospholipase Acivity and Eicosanoid Production in Atlantic Salmon (Salmo salar), Prostaglandins, Leukotrienes Essent. Fatty Acids 49, 665–673.
Wales, N.A.M. (1988) Hormone Studies in Myxine glutinosa: Effects of the Eicosanoids Arachidonic Acid, Prostaglandins E1, E2, A2, F2α, Thromboxane B2 and of Indomethacin on Plasma Cortisol, Blood Pressure, Urine Flow, and Electrolyte Balance, J. Comp. Physiol. B 158, 621–626.
Van Praag, D., Farber, S.J., Minkin, E., and Primor, N. (1987) Production of Eicosanoids by the Killifish Gills and Opercular Epithelia and Their Effect on Active Transport of Ions, Gen. Comp. Endocrinol. 67, 50–57.
Beckman, B., and Mustafa, T. (1992) Arachidonic Acid Metabolism in Gill Homogenate and Isolated Gill Cells from Rainbow Trout, Oncorhynchus mykiss: The Effect of Osmolality, Electrolytes, and Prolactin, Fish Physiol. Biochem. 10, 213–222.
Author information
Authors and Affiliations
About this article
Cite this article
Bell, J.G., Tocher, D.R., Farndale, B.M. et al. The effect of dietary lipid on polyunsaturated fatty acid metabolism in Atlantic salmon (Salmo salar) undergoing parr-smolt transformation. Lipids 32, 515–525 (1997). https://doi.org/10.1007/s11745-997-0066-4
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-997-0066-4