Abstract
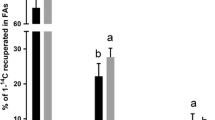
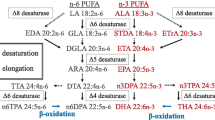
The aim of the present study was to investigate how EPA, DHA, and lipoic acid (LA) influence the different metabolic steps in the n-3 fatty acid (FA) biosynthetic pathway in hepatocytes from Atlantic salmon fed four dietary levels (0, 0.5, 1.0 and 2.0%) of EPA, DHA or a 1:1 mixture of these FA. The hepatocytes were incubated with [1-14C] 18:3n-3 in the presence or absence of LA (0.2 mM). Increased endogenous levels of EPA and/or DHA and LA exposure both led to similar responses in cells with reduced desaturation and elongation of [1-14C] 18:3n-3 to 18:4n-3, 20:4n-3, and EPA, in agreement with reduced expression of the Δ6 desaturase gene involved in the first step of conversion. DHA production, on the other hand, was maintained even in groups with high endogenous levels of DHA, possibly due to a more complex regulation of this last step in the n-3 metabolic pathway. Inhibition of the Δ6 desaturase pathway led to increased direct elongation to 20:3n-3 by both DHA and LA. Possibly the route by 20:3n-3 and then Δ8 desaturation to 20:4n-3, bypassing the first Δ6 desaturase step, can partly explain the maintained or even increased levels of DHA production. LA increased DHA production in the phospholipid fraction of hepatocytes isolated from fish fed 0 and 0.5% EPA and/or DHA, indicating that LA has the potential to further increase the production of this health-beneficial FA in fish fed diets with low levels of EPA and/or DHA.




Similar content being viewed by others
Abbreviations
- ASP:
-
Acid-soluble products
- CE:
-
Cholesteryl esters
- DHA:
-
Docosahexaenoic acid (22:6n-3)
- EPA:
-
Eicosapentaenoic acid (20:5n-3)
- FO:
-
Fish oil
- LA:
-
Lipoic acid
- MDG:
-
Monoacylglycerols and diacylglycerols
- NL:
-
Neutral lipids
- PL:
-
Phospholipids
- PUFA:
-
Polyunsaturated fatty acid(s)
- TAG:
-
Triacylglycerol(s)
- LC-PUFA:
-
Long chain polyunsaturated fatty acid(s)
- VO:
-
Vegetable oil
References
Ytrestøyl T, Aas TS, Åsgård T (2015) Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture 448:365–374
Tocher DR (2015) Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449:94–107
Tocher DR, Leaver MJ, Hodgson PA (1998) Recent advances in the biochemistry and molecular biology of fatty acyl desaturases. Prog Lipid Res 37:73–117
Castro LF, Tocher DR, Monroig O (2016) Long-chain polyunsaturated fatty acid biosynthesis in chordates: insights into the evolution of Fads and Elovl gene repertoire. Prog Lipid Res 62:25–40
Ruyter B, Røsjø C, Einen O, Thomassen MS (2000) Essential fatty acids in Atlantic salmon: time course of changes in fatty acid composition of liver, blood and carcass induced by a diet deficient in n-3 and n-6 fatty acids. Aquac Nutr 6:109–117
Ruyter B, Thomassen MS (1999) Metabolism of n-3 and n-6 fatty acids in Atlantic salmon liver: stimulation by essential fatty acid deficiency. Lipids 34:1167–1176
Zheng X, Tocher DR, Dickson CA, Bell JG, Teale AJ (2005) Highly unsaturated fatty acid synthesis in vertebrates: new insights with the cloning and characterization of a delta6 desaturase of Atlantic salmon. Lipids 40:13–24
Hastings N, Agaba MK, Tocher DR, Zheng X, Dickson CA, Dick JR, Teale AJ (2004) Molecular cloning and functional characterization of fatty acyl desaturase and elongase cDNAs involved in the production of eicosapentaenoic and docosahexaenoic acids from alpha-linolenic acid in Atlantic salmon (Salmo salar). Mar Biotechnol (New York, NY) 6:463–474
Monroig O, Zheng X, Morais S, Leaver MJ, Taggart JB, Tocher DR (2010) Multiple genes for functional 6 fatty acyl desaturases (Fad) in Atlantic salmon (Salmo salar L.): gene and cDNA characterization, functional expression, tissue distribution and nutritional regulation. Biochim Biophys Acta 1801:1072–1081
Carmona-Antonanzas G, Monroig O, Dick JR, Davie A, Tocher DR (2011) Biosynthesis of very long-chain fatty acids (C > 24) in Atlantic salmon: cloning, functional characterisation, and tissue distribution of an Elovl4 elongase. Comp Biochem Physiol B Biochem Mol Biol 159:122–129
Morais S, Monroig O, Zheng X, Leaver MJ, Tocher DR (2009) Highly unsaturated fatty acid synthesis in Atlantic salmon: characterization of ELOVL5- and ELOVL2-like elongases. Mar Biotechnol (New York, NY) 11:627–639
Monroig Ó, Li Y, Tocher DR (2011) Delta-8 desaturation activity varies among fatty acyl desaturases of teleost fish: high activity in delta-6 desaturases of marine species. Comp Biochem Physiol B Biochem Mol Biol 159:206–213
Park WJ, Kothapalli KS, Lawrence P, Tyburczy C, Brenna JT (2009) An alternate pathway to long-chain polyunsaturates: the FADS2 gene product Delta8-desaturates 20:2n-6 and 20:3n-3. J Lipid Res 50:1195–1202
Sprecher H (2000) Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids 1486:219–231
Li Y, Monroig O, Zhang L, Wang S, Zheng X, Dick JR, You C, Tocher DR (2010) Vertebrate fatty acyl desaturase with Δ4 activity. Proc Natl Acad Sci 107:16840–16845
Morais S, Castanheira F, Martinez-Rubio L, Conceicao LE, Tocher DR (2012) Long chain polyunsaturated fatty acid synthesis in a marine vertebrate: ontogenetic and nutritional regulation of a fatty acyl desaturase with Delta4 activity. Biochim Biophys Acta 1821:660–671
Kuah MK, Jaya-Ram A, Shu-Chien AC (2015) The capacity for long-chain polyunsaturated fatty acid synthesis in a carnivorous vertebrate: functional characterisation and nutritional regulation of a Fads2 fatty acyl desaturase with Delta4 activity and an Elovl5 elongase in striped snakehead (Channa striata). Biochim Biophys Acta 1851:248–260
Fonseca-Madrigal J, Navarro JC, Hontoria F, Tocher DR, Martínez-Palacios CA, Monroig Ó (2014) Diversification of substrate specificities in teleostei Fads2: characterization of Δ4 and Δ6Δ5 desaturases of Chirostoma estor. J Lipid Res 55:1408–1419
Morais S, Mourente G, Martinez A, Gras N, Tocher DR (2015) Docosahexaenoic acid biosynthesis via fatty acyl elongase and Delta4-desaturase and its modulation by dietary lipid level and fatty acid composition in a marine vertebrate. Biochim Biophys Acta 1851:588–597
Park HG, Park WJ, Kothapalli KS, Brenna JT (2015) The fatty acid desaturase 2 (FADS2) gene product catalyzes Delta4 desaturation to yield n-3 docosahexaenoic acid and n-6 docosapentaenoic acid in human cells. FASEB J Off Publ Fed Am Soc Exp Biol 29:3911–3919
Tocher DR, Bell JG, Dick JR, Sargent JR (1997) Fatty acyl desaturation in isolated hepatocytes from Atlantic salmon (Salmo salar): stimulation by dietary borage oil containing gamma-linolenic acid. Lipids 32:1237–1247
Tocher DR, Bell JG, MacGlaughlin P, McGhee F, Dick JR (2001) Hepatocyte fatty acid desaturation and polyunsaturated fatty acid composition of liver in salmonids: effects of dietary vegetable oil. Comp Biochem Physiol B Biochem Mol Biol 130:257–270
Carmona-Antonanzas G, Tocher DR, Martinez-Rubio L, Leaver MJ (2014) Conservation of lipid metabolic gene transcriptional regulatory networks in fish and mammals. Gene 534:1–9
Zheng X, Torstensen BE, Tocher DR, Dick JR, Henderson RJ, Bell JG (2005) Environmental and dietary influences on highly unsaturated fatty acid biosynthesis and expression of fatty acyl desaturase and elongase genes in liver of Atlantic salmon (Salmo salar). Biochim Biophys Acta 1734:13–24
Tocher DR, Bell JG, Dick JR, Crampton VO (2003) Effects of dietary vegetable oil on Atlantic salmon hepatocyte fatty acid desaturation and liver fatty acid compositions. Lipids 38:723–732
Thomassen MS, Rein D, Berge GM, Østbye TK, Ruyter B (2012) High dietary EPA does not inhibit ∆5 and ∆6 desaturases in Atlantic salmon (Salmo salar L.) fed rapeseed oil diets. Aquaculture 360:78–85
Leaver MJ, Bautista JM, Björnsson BT, Jönsson E, Krey G, Tocher DR, Torstensen BE (2008) Towards fish lipid nutrigenomics: current state and prospects for fin-fish aquaculture. Rev Fish Sci 16:73–94
Betancor MB, Sprague M, Sayanova O, Usher S, Campbell PJ, Napier JA, Caballero MJ, Tocher DR (2015) Evaluation of a high-EPA oil from transgenic Camelina sativa in feeds for Atlantic salmon (Salmo salar L.): effects on tissue fatty acid composition, histology and gene expression. Aquaculture 444:1–12
Brenna JT, Salem N Jr, Sinclair AJ, Cunnane SC (2009) α-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids 80:85–91
Prieto-Hontoria PL, Perez-Matute P, Fernandez-Galilea M, Bustos M, Martinez JA, Moreno-Aliaga MJ (2011) Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach. Biochim Biophys Acta 1807:664–678
Bast A, Haenen GR (2003) Lipoic acid: a multifunctional antioxidant. BioFactors (Oxford, England) 17:207–213
Trattner S, Pickova J, Park KH, Rinchard J, Dabrowski K (2007) Effects of α-lipoic and ascorbic acid on the muscle and brain fatty acids and antioxidant profile of the South American pacu Piaractus mesopotamicus. Aquaculture 273:158–164
Council NR (2011) Nutrient requirements of fish and shrimp. National academies press, Washington
Bou M, Berge GM, Baeverfjord G, Sigholt T, Østbye TK, Romarheim OH, Hatlen B, Leeuwis R, Venegas C, Ruyter B (Manuscript accepted) Requirements of omega-3 very long-chain polyunsaturated fatty acids in Atlantic salmon (Salmo salar L): effects of different dietary levels of EPA and DHA on fish performance and tissue composition and integrity. Br J Nutr
Dannevig BH, Berg T (1985) Endocytosis of galactose-terminated glycoproteins by isolated liver cells of the rainbow trout (Salmo gairdneri). Comp Biochem Physiol B Comp Biochem 82:683–688
Seglen PO (1976) Preparation of isolated rat liver cells. Methods Cell Biol 13:29–83
Kjær MA, Vegusdal A, Gjøen T, Rustan AC, Todorčević M, Ruyter B (2008) Effect of rapeseed oil and dietary n-3 fatty acids on triacylglycerol synthesis and secretion in Atlantic salmon hepatocytes. Biochim Biophys Acta 1781:112–122
Schiller Vestergren AL, Trattner S, Mraz J, Ruyter B, Pickova J (2011) Fatty acids and gene expression responses to bioactive compounds in Atlantic salmon (Salmo salar L.) hepatocytes. Neuro Endocrinol Lett 32(Suppl 2):41–50
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Mason ME, Waller GR (1964) Dimethoxypropane induced transesterification of fats and oils in preparation of methyl esters for gas chromatographic analysis. Anal Chem 36:583–586
Hoshi M, Williams M, Kishimoto Y (1973) Esterification of fatty acids at room temperature by chloroform-methanolic HCl-cupric acetate. J Lipid Res 14:599–601
Narce M, Gresti J, Bezard J (1988) Method for evaluating the bioconversion of radioactive polyunsaturated fatty acids by use of reversed-phase liquid chromatography. J Chromatogr 448:249–264
Christiansen R, Borrebaek B, Bremer J (1976) The effect of (−)carnitine on the metabolism of palmitate in liver cells isolated from fasted and refed rats. FEBS Lett 62:313–317
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Randall KM, Drew MD, Øverland M, Østbye TK, Bjerke M, Vogt G, Ruyter B (2013) Effects of dietary supplementation of coriander oil, in canola oil diets, on the metabolism of [1-14C] 18:3n-3 and [1-14C] 18:2n-6 in rainbow trout hepatocytes. Comp Biochem Physiol B Biochem Mol Biol 166:65–72
Stubhaug I, Tocher DR, Bell JG, Dick JR, Torstensen BE (2005) Fatty acid metabolism in Atlantic salmon (Salmo salar L.) hepatocytes and influence of dietary vegetable oil. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids 1734:277–288
Fonseca-Madrigal J, Bell JG, Tocher DR (2006) Nutritional and environmental regulation of the synthesis of highly unsaturated fatty acids and of fatty-acid oxidation in Atlantic salmon (Salmo salar L.) enterocytes and hepatocytes. Fish Physiol Biochem 32:317–328
Ruyter B, Røsjø C, Grisdale-Helland B, Rosenlund G, Obach A, Thomassen MS (2003) Influence of temperature and high dietary linoleic acid content on esterification, elongation, and desaturation of PUFA in Atlantic salmon hepatocytes. Lipids 38:833–840
Stanković MN, Mladenović D, Ninković M, Ðuričić I, Šobajić S, Jorgačević B, de Luka S, Vukicevic RJ, Radosavljević TS (2014) The effects of α-lipoic acid on liver oxidative stress and free fatty acid composition in methionine-choline deficient diet-induced NAFLD. J Med Food 17:254–261
Trattner S, Ruyter B, Østbye TK, Gjøen T, Zlabek V, Kamal-Eldin A, Pickova J (2008) Sesamin increases alpha-linolenic acid conversion to docosahexaenoic acid in Atlantic salmon (Salmo salar L.) hepatocytes: role of altered gene expression. Lipids 43:999–1008
Betancor MB, Olsen RE, Solstorm D, Skulstad OF, Tocher DR (2016) Assessment of a land-locked Atlantic salmon (Salmo salar L.) population as a potential genetic resource with a focus on long-chain polyunsaturated fatty acid biosynthesis. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids 1861:227–238
Espenshade PJ (2006) SREBPs: sterol-regulated transcription factors. J Cell Sci 119:973–976
Moya-Falcón C, Thomassen MS, Jakobsen JV, Ruyter B (2005) Effects of dietary supplementation of rapeseed oil on metabolism of [1-14C] 18:1n-9, [1-14C] 20:3n-6, and [1-14C] 20:4n-3 in Atlantic salmon hepatocytes. Lipids 40:709–717
Vegusdal A, Ostbye TK, Tran TN, Gjoen T, Ruyter B (2004) Beta-oxidation, esterification, and secretion of radiolabeled fatty acids in cultivated Atlantic salmon skeletal muscle cells. Lipids 39:649–658
Pashaj A, Xia M, Moreau R (2015) Alpha-lipoic acid as a triglyceride-lowering nutraceutical. Can J Physiol Pharmacol 93:1029–1041
Butler JA, Hagen TM, Moreau R (2009) Lipoic acid improves hypertriglyceridemia by stimulating triacylglycerol clearance and downregulating liver triacylglycerol secretion. Arch Biochem Biophys 485:63–71
Seo EY, Ha AW, Kim WK (2012) α-Lipoic acid reduced weight gain and improved the lipid profile in rats fed with high fat diet. Nutr Res Pract 6:195–200
RL Yang, Li W, Shi YH, Le GW (2008) Lipoic acid prevents high-fat diet–induced dyslipidemia and oxidative stress: a microarray analysis. Nutrition 24:582–588
Lee WJ, Song KH, Koh EH, Won JC, Kim HS, Park HS, Kim MS, Kim SW, Lee KU, Park JY (2005) Alpha-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem and Biophys Res Commun 332:885–891
Walgren JL, Amani Z, McMillan JM, Locher M, Buse MG (2004) Effect of R(+)α-lipoic acid on pyruvate metabolism and fatty acid oxidation in rat hepatocytes. Metabolism 53:165–173
Acknowledgements
The authors would like to thank Målfrid Tofteberg Bjerke and Inger Øien Kristiansen for their skilful technical assistance. This work was carried out with support from the Norwegian Research Council (Grant Number NFR 224913).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to report.
About this article
Cite this article
Bou, M., Østbye, TK., Berge, G.M. et al. EPA, DHA, and Lipoic Acid Differentially Modulate the n-3 Fatty Acid Biosynthetic Pathway in Atlantic Salmon Hepatocytes. Lipids 52, 265–283 (2017). https://doi.org/10.1007/s11745-017-4234-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-017-4234-5