Abstract
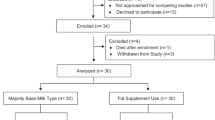
Docosahexaenoic acid (DHA) is an essential fatty acid (FA) important for health and neurodevelopment. Premature infants are at risk of DHA deficiency and circulating levels directly correlate with health outcomes. Most supplementation strategies have focused on increasing DHA content in mother’s milk or infant formula. However, extremely premature infants may not reach full feedings for weeks and commercially available parenteral lipid emulsions do not contain preformed DHA, so blood levels decline rapidly after birth. Our objective was to develop a DHA supplementation strategy to overcome these barriers. This double-blind, randomized, controlled trial determined feasibility, tolerability and efficacy of daily enteral DHA supplementation (50 mg/day) in addition to standard nutrition for preterm infants (24–34 weeks gestational age) beginning in the first week of life. Blood FA levels were analyzed at baseline, full feedings and near discharge in DHA (n = 31) or placebo supplemented (n = 29) preterm infants. Term peers (n = 30) were analyzed for comparison. Preterm infants had lower baseline DHA levels (p < 0.0001). Those receiving DHA had a progressive increase in circulating DHA over time (from 3.33 to 4.09 wt% or 2.88 to 3.55 mol%, p < 0.0001) while placebo-supplemented infants (receiving standard neonatal nutrition) had no increase over time (from 3.35 to 3.32 wt% or 2.91 to 2.87 mol%). Although levels increased with additional DHA supplementation, preterm infants still had lower blood DHA levels than term peers (4.97 wt% or 4.31 mol%) at discharge (p = 0.0002). No differences in adverse events were observed between the groups. Overall, daily enteral DHA supplementation is feasible and alleviates deficiency in premature infants.


Similar content being viewed by others
Abbreviations
- ALA:
-
α-Linolenic acid
- ARA:
-
Arachidonic acid
- BPD:
-
Bronchopulmonary dysplasia
- DSMB:
-
Data safety and monitoring board
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acid(s)
- GA:
-
Gestational age
- IUGR:
-
Intrauterine growth restriction
- LNA:
-
Linoleic acid
- LCPUFA:
-
Long chain polyunsaturated fatty acid(s)
- NEC:
-
Necrotizing enterocolitis
- NICU:
-
Neonatal intensive care unit
- ROP:
-
Retinopathy of prematurity
References
Kuipers RS, Luxwolda MF, Offringa PJ, Boersma ER, Dijck-Brouwer DA, Muskiet FA (2012) Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot Essent Fatty Acids 86:13–20
Innis SM (2005) Essential fatty acid transfer and fetal development. Placenta 26 Suppl A:S70–S75
Harris WS, Baack ML (2015) Beyond building better brains: bridging the docosahexaenoic acid (DHA) gap of prematurity. J Perinatol 35:1–7
Lapillonne AJC (2009) Reevaluation of the DHA requirement for the premature infant. Prostaglandins Leukot Essent Fatty Acids 143–150
Baack ML, Puumala SE, Messier SE, Pritchett DK, Harris WS (2015) What is the relationship between gestational age and docosahexaenoic acid (DHA) and arachidonic acid (ARA) levels? Prostaglandins Leukot Essent Fatty Acids 100:5–11
Lapillonne A, Eleni dit Trolli S, Kermorvant-Duchemin E (2010) Postnatal docosahexaenoic acid deficiency is an inevitable consequence of current recommendations and practice in preterm infants. Neonatology 98:397–403
Carlson SE, Montalto MB, Ponder DL, Werkman SH, Korones SB (1998) Lower incidence of necrotizing enterocolitis in infants fed a preterm formula with egg phospholipids. Pediatr Res 44:491–498
Pawlik D, Lauterbach R, Walczak M, Hurkala J, Sherman MP (2014) Fish-oil fat emulsion supplementation reduces the risk of retinopathy in very low birth weight infants: a prospective, randomized study. JPEN J Parenter Enteral Nutr 38:711–716
Martin CR, Dasilva DA, Cluette-Brown JE, Dimonda C, Hamill A, Bhutta AQ, Coronel E, Wilschanski M, Stephens AJ, Driscoll DF et al (2011) Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J Pediatr 159(743–749):e741–742
Manley BJ, Makrides M, Collins CT, McPhee AJ, Gibson RA, Ryan P, Sullivan TR, Davis PG (2011) High-dose docosahexaenoic acid supplementation of preterm infants: respiratory and allergy outcomes. Pediatrics 128:e71–77
Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, Simmer K, Colditz PB, Morris S, Smithers LG et al (2009) Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA 301:175–182
Zhang P, Lavoie PM, Lacaze-Masmonteil T, Rhainds M, Marc I (2014) Omega-3 long-chain polyunsaturated fatty acids for extremely preterm infants: a systematic review. Pediatrics 134:120–134
Brenna JT (2002) Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr Opin Clin Nutr Metab Care 5:127–132
Collins CT, Sullivan TR, McPhee AJ, Stark MJ, Makrides M, Gibson RA (2015) A dose response randomised controlled trial of docosahexaenoic acid (DHA) in preterm infants. Prostaglandins Leukot Essent Fatty Acids 99:1–6
Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM (2007) Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr 85:1457–1464
Fenton TR, Nasser R, Eliasziw M, Kim JH, Bilan D, Sauve R (2013) Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr 13:92
Group WMGRS: WHO Child Growth Standards (2009) Growth velocity based on weight, length and head circumference: methods and development, p 242
Verbeke G, Molenbergh G (2000) Linear mixed models for longitudinal data. Springer-Verlag, New York
Lucas A, Morley R, Cole TJ, Lister G, Leeson-Payne C (1992) Breast milk and subsequent intelligence quotient in children born preterm. Lancet 339:261–264
Agostoni C, Marangoni F, Lammardo AM, Giovannini M, Riva E, Galli C (2001) Breastfeeding duration, milk fat composition and developmental indices at 1 year of life among breastfed infants. Prostaglandins Leukot Essent Fatty Acids 64:105–109
Jensen CL, Voigt RG, Prager TC, Zou YL, Fraley JK, Rozelle JC, Turcich MR, Llorente AM, Anderson RE, Heird WC (2005) Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am J Clin Nutr 82:125–132
Lauritzen L, Jorgensen MH, Mikkelsen TB, Skovgaard M, Straarup EM, Olsen SF, Hoy CE, Michaelsen KF (2004) Maternal fish oil supplementation in lactation: effect on visual acuity and n-3 fatty acid content of infant erythrocytes. Lipids 39:195–206
Gibson RA, Neumann MA, Makrides M (1997) Effect of increasing breast milk docosahexaenoic acid on plasma and erythrocyte phospholipid fatty acids and neural indices of exclusively breast fed infants. Eur J Clin Nutr 51:578–584
Lauritzen L, Jorgensen MH, Olsen SF, Straarup EM, Michaelsen KF (2005) Maternal fish oil supplementation in lactation: effect on developmental outcome in breast-fed infants. Reprod Nutr Dev 45:535–547
Makrides M, Neumann MA, Gibson RA (1996) Effect of maternal docosahexaenoic acid (DHA) supplementation on breast milk composition. Eur J Clin Nutr 50:352–357
Auestad N, Halter R, Hall RT, Blatter M, Bogle ML, Burks W, Erickson JR, Fitzgerald KM, Dobson V, Innis SM et al (2001) Growth and development in term infants fed long-chain polyunsaturated fatty acids: a double-masked, randomized, parallel, prospective, multivariate study. Pediatrics 108:372–381
Birch EE, Garfield S, Castaneda Y, Hughbanks-Wheaton D, Uauy R, Hoffman D (2007) Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev 83:279–284
Innis SM, Adamkin DH, Hall RT, Kalhan SC, Lair C, Lim M, Stevens DC, Twist PF, Diersen-Schade DA, Harris CL et al (2002) Docosahexaenoic acid and arachidonic acid enhance growth with no adverse effects in preterm infants fed formula. J Pediatr 140:547–554
Smithers LG, Gibson RA, McPhee A, Makrides M (2008) Effect of two doses of docosahexaenoic acid (DHA) in the diet of preterm infants on infant fatty acid status: results from the DINO trial. Prostaglandins Leukot Essent Fatty Acids 79:141–146
Vanderhoof J, Gross S, Hegyi T, Clandinin T, Porcelli P, DeCristofaro J, Rhodes T, Tsang R, Shattuck K, Cowett R et al (1999) Evaluation of a long-chain polyunsaturated fatty acid supplemented formula on growth, tolerance, and plasma lipids in preterm infants up to 48 weeks postconceptional age. J Pediatr Gastroenterol Nutr 29:318–326
Marseglia L, D’Angelo G, Manti S, Arrigo T, Barberi I, Reiter RJ, Gitto E (2014) Oxidative stress-mediated aging during the fetal and perinatal periods. Oxid Med Cell Longev 2014:358375
Mohamed I, Elremaly W, Rouleau T, Lavoie JC (2015) Oxygen and parenteral nutrition two main oxidants for extremely preterm infants: ‘it all adds up’. J Neonatal Perinatal Med 8:189–197
Bazan NG, Molina MF, Gordon WC (2011) Docosahexaenoic Acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases. Annu Rev Nutr 31:321–351
Liu Q, Wu D, Ni N, Ren H, Luo C, He C, Kang JX, Wan JB, Su H (2014) Omega-3 polyunsaturated fatty acids protect neural progenitor cells against oxidative injury. Mar Drugs 12:2341–2356
Lu J, Jilling T, Li D, Caplan MS (2007) Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Pediatr Res 61:427–432
Beken S, Dilli D, Fettah ND, Kabatas EU, Zenciroglu A, Okumus N (2014) The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial. Early Hum Dev 90:27–31
Pawlik D, Lauterbach R, Walczak M, Hurkala J (2011) Docosahexaenoic acid (DHA) concentration in very low birth weight newborns receiving a fish-oil based fat emulsion from the first day of life. Preliminary clinical observation. Med Wieku Rozwoj 15:312–317
Henriksen C, Haugholt K, Lindgren M, Aurvag AK, Ronnestad A, Gronn M, Solberg R, Moen A, Nakstad B, Berge RK et al (2008) Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 121:1137–1145
Lien VW, Clandinin MT (2009) Dietary assessment of arachidonic acid and docosahexaenoic acid intake in 4–7 year-old children. J Am Coll Nutr 28:7–15
Tomsits E, Pataki M, Tolgyesi A, Fekete G, Rischak K, Szollar L (2010) Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, and fish oil: a randomised, double-blind clinical trial in premature infants requiring parenteral nutrition. J Pediatr Gastroenterol Nutr 51:514–521
Lien EL (2009) Toxicology and safety of DHA. Prostaglandins Leukot Essent Fatty Acids 81:125–132
Puder M, Valim C, Meisel JA, Le HD, de Meijer VE, Robinson EM, Zhou J, Duggan C, Gura KM (2009) Parenteral fish oil improves outcomes in patients with parenteral nutrition-associated liver injury. Ann Surg 250:395–402
Rollins MD, Scaife ER, Jackson WD, Meyers RL, Mulroy CW, Book LS (2010) Elimination of soybean lipid emulsion in parenteral nutrition and supplementation with enteral fish oil improve cholestasis in infants with short bowel syndrome. Nutr Clin Pract 25:199–204
Tillman EM, Crill CM, Black DD, Hak EB, Lazar LF, Christensen ML, Huang EY, Helms RA (2011) Enteral fish oil for treatment of parenteral nutrition-associated liver disease in six infants with short-bowel syndrome. Pharmacotherapy 31:503–509
Phang M, Lincz LF, Garg ML (2013) Eicosapentaenoic and docosahexaenoic acid supplementations reduce platelet aggregation and hemostatic markers differentially in men and women. J Nutr 143:457–463
Carlson SE, Cooke RJ, Werkman SH, Tolley EA (1992) First year growth of preterm infants fed standard compared to marine oil n-3 supplemented formula. Lipids 27:901–907
Acknowledgments
We gratefully acknowledges our exceptional study team (Von “Mimi” Chau, Diane Hahn, Katherine Burgess, Dawn Hansen, Jessica Howard, Carmen Sandman, Melissa Hemenway), DSMB members (Pam Koepsell, Dr. Lisa Jaton, Dr. Paul Thompson, Dr. Dennis Stevens, Michele Sturdevant, and Dr. Amanda Jensen), NICU staff nurses, pharmacy staff (Linda Oyen), neonatologists, and especially our NICU babies and their families—it is such a privilege to care for you. Funding for this study was provided by a Sanford Health Seed Grant, a Gerber Foundation Pediatric Nutrition Grant (PN12-005-1372-3069), and Sanford Research. Dr. Baack also receives support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (K08HD078504) and an NIH Center of Biomedical Research Excellence (COBRE) Grant (P20 GM103620-01A1). Further information about this trial and ongoing efforts may be found at ClinicalTrials.gov (NCT01908907).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. William S. Harris who serves as a senior mentor to Dr. Baack, is the Founder and President of OmegaQuant, LLC, but has no foreseen financial gain from publication of this work. Additional authors declare no conflict of interest. Funding sponsors had no role in study design, implementation, data collection, analyses, interpretation or publication.
About this article
Cite this article
Baack, M.L., Puumala, S.E., Messier, S.E. et al. Daily Enteral DHA Supplementation Alleviates Deficiency in Premature Infants. Lipids 51, 423–433 (2016). https://doi.org/10.1007/s11745-016-4130-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-016-4130-4