Abstract
Unsaturated free fatty acids (FFA) are able to prevent deleterious effects of saturated FFA in skeletal muscle cells although the mechanisms involved are still not completely understood. FFA act as endogenous ligands of peroxisome proliferator-activated receptors (PPAR), transcription factors regulating the expression of genes involved in lipid metabolism. The aim of this study was to determine whether activation of PPARδ, the most common PPAR subtype in skeletal muscle, plays a role in mediating the protective effect of unsaturated FFA on saturated FFA-induced damage in skeletal muscle cells and to examine an impact on mitochondrial respiration. Mouse C2C12 myotubes were treated for 24 h with different concentrations of saturated FFA (palmitic acid), unsaturated FFA (oleic, linoleic and α-linolenic acid), and their combinations. PPARδ agonist GW501516 and antagonist GSK0660 were also used. Both mono- and polyunsaturated FFA, but not GW501516, prevented palmitic acid-induced cell death. Mono- and polyunsaturated FFA proved to be effective activators of PPARδ compared to saturated palmitic acid; however, in combination with palmitic acid their effect on PPARδ activation was blocked and stayed at the levels observed for palmitic acid alone. Unsaturated FFA at moderate physiological concentrations as well as GW501516, but not palmitic acid, mildly uncoupled mitochondrial respiration. Our results indicate that although unsaturated FFA are effective activators of PPARδ, their protective effect on palmitic acid-induced toxicity is not mediated by PPARδ activation and subsequent induction of lipid regulatory genes in skeletal muscle cells. Other mechanisms, such as mitochondrial uncoupling, may underlie their effect.






Similar content being viewed by others
Abbreviations
- FFA:
-
Free fatty acids
- PPAR:
-
Peroxisome proliferator-activated receptors
- PDK4:
-
Pyruvate dehydrogenase kinase 4
- UCP3:
-
Uncoupling protein 3
- CPT1:
-
Carnitine palmitoyltransferase 1
References
Yuzefovych LV, Solodushko VA, Wilson GL, Rachek LI (2012) Protection from palmitate-induced mitochondrial DNA damage prevents from mitochondrial oxidative stress, mitochondrial dysfunction, apoptosis, and impaired insulin signaling in rat L6 skeletal muscle cells. Endocrinology 153:92–100. doi:10.1210/en.2011-1442
Coll T, Eyre E, Rodríguez-Calvo R et al (2008) Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J Biol Chem 283:11107–11116. doi:10.1074/jbc.M708700200
Patková J, Anděl M, Trnka J (2014) Palmitate-induced cell death and mitochondrial respiratory dysfunction in myoblasts are not prevented by mitochondria-targeted antioxidants. Cell Physiol Biochem 33:1439–1451. doi:10.1159/000358709
Henique C, Mansouri A, Fumey G et al (2010) Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J Biol Chem 285:36818–36827. doi:10.1074/jbc.M110.170431
Meshkani R, Sadeghi A, Taheripak G et al (2014) Rosiglitazone, a PPARγ agonist, ameliorates palmitate-induced insulin resistance and apoptosis in skeletal muscle cells. Cell Biochem Funct 32:683–691. doi:10.1002/cbf.3072
Tumova J, Andel M, Trnka J (2015) Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol Res (in press)
Hirabara SM, Curi R, Maechler P (2010) Saturated fatty acid-induced insulin resistance is associated with mitochondrial dysfunction in skeletal muscle cells. J Cell Physiol 222:187–194. doi:10.1002/jcp.21936
Yuzefovych L, Wilson G, Rachek L (2010) Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab 299:E1096–E1105. doi:10.1152/ajpendo.00238.2010
Lam YY, Hatzinikolas G, Weir JM et al (2011) Insulin-stimulated glucose uptake and pathways regulating energy metabolism in skeletal muscle cells: the effects of subcutaneous and visceral fat, and long-chain saturated, n-3 and n-6 polyunsaturated fatty acids. Biochim Biophys Acta 1811:468–475. doi:10.1016/j.bbalip.2011.04.011
Pickersgill L, Litherland GJ, Greenberg AS et al (2007) Key role for ceramides in mediating insulin resistance in human muscle cells. J Biol Chem 282:12583–12589. doi:10.1074/jbc.M611157200
Szendroedi J, Yoshimura T, Phielix E et al (2014) Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc Natl Acad Sci USA 111:9597–9602. doi:10.1073/pnas.1409229111
Perdomo G, Commerford SR, Richard AM et al (2004) Increased beta-oxidation in muscle cells enhances insulin-stimulated glucose metabolism and protects against fatty acid-induced insulin resistance despite intramyocellular lipid accumulation. J Biol Chem 279:27177–27186. doi:10.1074/jbc.M403566200
Forman BM, Chen J, Evans RM (1997) Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci USA 94:4312–4317
Braissant O, Foufelle F, Scotto C et al (1996) Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology 137:354–366. doi:10.1210/endo.137.1.8536636
Muoio DM, MacLean PS, Lang DB et al (2002) Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. J Biol Chem 277:26089–26097. doi:10.1074/jbc.M203997200
Ehrenborg E, Krook A (2009) Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor delta. Pharmacol Rev 61:373–393. doi:10.1124/pr.109.001560
Nakamura MT, Yudell BE, Loor JJ (2014) Regulation of energy metabolism by long-chain fatty acids. Prog Lipid Res 53:124–144. doi:10.1016/j.plipres.2013.12.001
Dressel U, Allen TL, Pippal JB et al (2003) The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol 17:2477–2493. doi:10.1210/me.2003-0151
Tanaka T, Yamamoto J, Iwasaki S et al (2003) Activation of peroxisome proliferator-activated receptor delta induces fatty acid beta-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci USA 100:15924–15929. doi:10.1073/pnas.0306981100
Krämer DK, Al-Khalili L, Guigas B et al (2007) Role of AMP kinase and PPARδ in the regulation of lipid and glucose metabolism in human skeletal muscle. J Biol Chem 282:19313–19320. doi:10.1074/jbc.M702329200
Coll T, Alvarez-Guardia D, Barroso E et al (2010) Activation of peroxisome proliferator-activated receptor-delta by GW501516 prevents fatty acid-induced nuclear factor-{kappa}B activation and insulin resistance in skeletal muscle cells. Endocrinology 151:1560–1569. doi:10.1210/en.2009-1211
Cousin SP, Hügl SR, Wrede CE et al (2001) Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology 142:229–240
Brand MD, Nicholls DG (2011) Assessing mitochondrial dysfunction in cells. Biochem J 435:297–312. doi:10.1042/BJ20110162
Iwashita A, Muramatsu Y, Yamazaki T et al (2007) Neuroprotective efficacy of the peroxisome proliferator-activated receptor delta-selective agonists in vitro and in vivo. J Pharmacol Exp Ther 320:1087–1096. doi:10.1124/jpet.106.115758
Koves TR, Ussher JR, Noland RC et al (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7:45–56. doi:10.1016/j.cmet.2007.10.013
Rodríguez AM, Sánchez J, Tobaruela A et al (2010) Time-course effects of increased fatty acid supply on the expression of genes involved in lipid/glucose metabolism in muscle cells. Cell Physiol Biochem 25:337–346. doi:10.1159/000276566
Barbosa MR, Sampaio IH, Teodoro BG et al (2013) Hydrogen peroxide production regulates the mitochondrial function in insulin resistant muscle cells: effect of catalase overexpression. Biochim Biophys Acta 1832:1591–1604. doi:10.1016/j.bbadis.2013.04.029
Shearer BG, Steger DJ, Way JM et al (2008) Identification and characterization of a selective peroxisome proliferator-activated receptor beta/delta (NR1C2) antagonist. Mol Endocrinol 22:523–529. doi:10.1210/me.2007-0190
Salvadó L, Coll T, Gómez-Foix AM et al (2013) Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 56:1372–1382. doi:10.1007/s00125-013-2867-3
Cheon HG, Cho YS (2014) Protection of palmitic acid-mediated lipotoxicity by arachidonic acid via channeling of palmitic acid into triglycerides in C2C12. J Biomed Sci 21:13. doi:10.1186/1423-0127-21-13
Kadenbach B (2003) Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta 1604:77–94. doi:10.1016/S0005-2728(03)00027-6
Maassen JA, Romijn JA, Heine RJ (2007) Fatty acid-induced mitochondrial uncoupling in adipocytes as a key protective factor against insulin resistance and beta cell dysfunction: a new concept in the pathogenesis of obesity-associated type 2 diabetes mellitus. Diabetologia 50:2036–2041. doi:10.1007/s00125-007-0776-z
Konrad D, Rudich A, Bilan PJ et al (2005) Troglitazone causes acute mitochondrial membrane depolarisation and an AMPK-mediated increase in glucose phosphorylation in muscle cells. Diabetologia 48:954–966. doi:10.1007/s00125-005-1713-7
Hirabara SM, Silveira LR, Alberici LC et al (2006) Acute effect of fatty acids on metabolism and mitochondrial coupling in skeletal muscle. Biochim Biophys Acta 1757:57–66. doi:10.1016/j.bbabio.2005.11.007
Acknowledgments
This work was supported by the research project of Grant Agency of Charles University GAUK 661912 and grants PRVOUK-31 and UNCE 204015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11745_2015_4058_MOESM1_ESM.eps
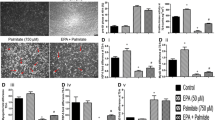
The mRNA expression of PPARδ. a, b) Myotubes were treated with 1 μM PPARδ agonist GW501516 (a) or 1 μM PPARδ antagonist GSK0660 (b) for 24 h. c, d, e) Myotubes were treated with 100 μM (c), 300 μM (d) and 600 μM (e) total concentration of palmitic acid (PAM), unsaturated FFA (OLA, LNA, ALA) or with combinations: PAM + unsaturated FFA in a ratio 1:1 or PAM + 1 μM GW501516 for 24 h. The mRNA level relative to the internal control GAPDH was determined by RT-PCR analysis and fold change of expression was calculated using the ΔΔCt method. Results are presented as mean ± SEM (n = 3). * p < 0.05 compared to BSA control (C-BSA) (EPS 788 kb).
11745_2015_4058_MOESM2_ESM.eps
Mitochondrial respiration (oxygen consumption rates, OCR) in myotubes measured under different respiratory conditions. a, b) Myotubes were treated with 100 μM (c), 300 μM (d) and 600 μM (e) total concentration of palmitic acid (PAM), unsaturated FFA (OLA, LNA, ALA) or with combinations: PAM + unsaturated FFA in a ratio 1:1 or PAM + 1 μM GW501516 for 24 h. c) Myotubes were treated with 1 μM PPARδ agonist GW501516 for 24 h. Basal and maximal respiration were assessed by a stress test as described in the Methods section. Results are presented as mean ± SEM (n ≥ 3). * p < 0.05 compared to control (C) (EPS 950 kb).
About this article
Cite this article
Tumova, J., Malisova, L., Andel, M. et al. Protective Effect of Unsaturated Fatty Acids on Palmitic Acid-Induced Toxicity in Skeletal Muscle Cells is not Mediated by PPARδ Activation. Lipids 50, 955–964 (2015). https://doi.org/10.1007/s11745-015-4058-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-015-4058-0