Abstract
Cardiovascular safety of cyclooxygenase (COX)-2-selective inhibitors and nonselective nonsteroidal anti-inflammatory drugs (NSAIDs) is of worldwide concern. COX-2 inhibitors and NSAIDs act by inhibiting arachidonic acid metabolism to prostaglandins. They confer a cardiovascular hazard manifested as an elevated risk of myocardial infarction. Mechanisms underlying these cardiovascular effects are uncertain. Here we determine whether interference with cytosolic phospholipase A2 (cPLA-2) or COX-2 through pharmacologic blockade or silencing RNA impacts expression of scavenger receptor CD36 and scavenger receptor A, both involved in cholesterol uptake in monocytes and macrophages. THP-1 human monocytes and human peripheral blood mononuclear cells were exposed to celecoxib, a COX-2 selective inhibitor currently in clinical use, and to arachidonyl trifluoromethyl ketone (AACOCF3), an arachidonic acid analog that selectively inhibits cPLA-2. Celecoxib and AACOCF3 each upregulated expression of CD36, but not scavenger receptor A, as determined by quantitative PCR and immunoblotting. Silencing of cPLA-2 or COX-2 had comparable effects to pharmacologic treatments. Oil red O staining revealed a profound increase in foam cell transformation of THP-1 macrophages exposed to either celecoxib or AACOCF3 (both 25 μM), supporting a role for the COX pathway in maintaining macrophage cholesterol homeostasis. Demonstration of disrupted cholesterol balance by AACOCF3 and celecoxib provides further evidence of the possible mechanism by which COX inhibition may promote lipid overload leading to atheromatous lesion formation and increased cardiovascular events.







Similar content being viewed by others
Abbreviations
- AACOCF3:
-
Arachidonyl trifluoromethyl ketone
- COX:
-
Cyclooxygenase
- cPLA-2:
-
Cytosolic phospholipase A2
- FCS:
-
Fetal calf serum
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- IFN:
-
Interferon
- HRP:
-
Horseradish peroxidase
- LDL:
-
Low density lipoprotein
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- PBMC:
-
Peripheral blood mononuclear cells
- PMA:
-
Phorbol 12-myristate 13-acetate
- RCT:
-
Reverse cholesterol transport
- ScR-A:
-
Scavenger receptor A
- TGF:
-
Transforming growth factor
References
Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G et al (2009) Heart disease and stroke statistics––2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 119:480–486
Yusuf S, Reddy S, Ounpuu S, Anand S (2001) Global burden of cardiovascular diseases part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 104:2746–2753
Goldstein JL, Brown MS (2009) The LDL receptor. Arterioscler Thromb Vasc Biol 29:431–438
Silverstein RL (2009) Inflammation, atherosclerosis, and arterial thrombosis: role of the scavenger receptor CD36. Cleve Clin J Med 76(Suppl 2):S27–S30
Platt N, Gordon S (2001) Is the class A macrophage scavenger receptor (SR-A) multifunctional?––The mouse’s tale. J Clin Invest 108:649–654
Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW (2002) Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem 277:49982–49988
Bunimov N, Laneuville O (2008) Cyclooxygenase inhibitors: instrumental drugs to understand cardiovascular homeostasis and arterial thrombosis. Cardiovasc Hematol Disord Drug Targets 8:268–277
Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ (2000) Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group N Engl J Med 343:1520–1528
Vardeny O, Solomon SD (2008) Cyclooxygenase-2 inhibitors, nonsteroidal anti-inflammatory drugs, and cardiovascular risk. Cardiol Clin 26:589–601
Wong D, Wang M, Cheng Y, FitzGerald GA (2005) Cardiovascular hazard and non-steroidal anti-inflammatory drugs. Curr Opin Pharmacol 5:204–210
Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FW, Schnitzer TJ et al (2004) Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomized controlled trial. Lancet 364:675–684
Ott E, Nussmeier NA, Duke PC, Feneck RO, Alston RP, Snabes MC, Hubbard RC, Hsu PH, Saidman LJ, Mangano DT (2003) Efficacy and safety of the cyclooxygenase-2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg 125:1481–1492
Dajani EZ, Islam K (2008) Cardiovascular and gastrointestinal toxicity of selective cyclo-oxygenase-2 inhibitors in man. J Physiol Pharmacol 59(Suppl 2):117–133
Chan ES, Zhang H, Fernandez P, Edelman SD, Pillinger MH, Ragolia L, Palaia T, Carsons S, Reiss AB (2007) Effect of COX inhibition on cholesterol efflux proteins and atheromatous foam cell transformation in THP-1 human macrophages: a possible mechanism for increased cardiovascular risk. Arthritis Res Ther 9:R4
Reiss AB, Anwar F, Chan ES, Anwar K (2009) Disruption of cholesterol efflux by coxib medications and inflammatory processes: link to increased cardiovascular risk. J Investig Med 57:695–702
Reiss AB, Rahman MM, Chan ES, Montesinos MC, Awadallah NW, Cronstein BN (2004) Adenosine A2A receptor occupancy stimulates expression of proteins involved in reverse cholesterol transport and inhibits foam cell formation in macrophages. J Leukoc Biol 76:727–734
Reiss AB, Patel CA, Rahman MM, Chan ES, Hasneen K, Montesinos MC, Trachman JD, Cronstein BN (2004) Interferon-gamma impedes reverse cholesterol transport and promotes foam cell transformation in THP-1 human monocytes/macrophages. Med Sci Monit 10(11):BR420–BR425
Reiss AB, Awadallah N, Malhotra S, Montesinos MC, Chan ESL, Javitt NB, Cronstein BN (2001) Immune complexes and interferon-γ decrease cholesterol 27-hydroxylase in human arterial endothelium and macrophages. J Lipid Res 42:1913–1922
Grainger DJ (2004) Transforming growth factor-β and atherosclerosis: so far, so good for the protective cytokine hypothesis. Arterioscler Thromb Vasc Biol 24:399–404
Draude G, Lorenz RL (2000) TGF-beta1 downregulates CD36 and scavenger receptor A but upregulates LOX-1 in human macrophages. Am J Physiol Heart Circ Physiol 278:H1042–H1048
Basu GD, Pathangey LB, Tinder TL, Gendler SJ, Mukherjee P (2005) Mechanisms underlying the growth inhibitory effects of the cyclo-oxygenase-2 inhibitor celecoxib in human breast cancer cells. Breast Cancer Res 7(4):R422–R435
Lee EJ, Kim SH, Kwark YE, Kim J (2006) Heterogeneous nuclear ribonuclear protein C is increased in the celecoxib-induced growth inhibition of human oral squamous cell carcinoma. Exp Mol Med 38(3):203–209
Pietsch A, Erl W, Lorenz RL (1996) Lovastatin reduces expression of the combined adhesion and scavenger receptor CD36 in human monocytic cells. Biochem Pharmacol 52:433–439
Han S, Sidell N (2002) Peroxisome-proliferator-activated-receptor gamma (PPARgamma) independent induction of CD36 in THP-1 monocytes by retinoic acid. Immunology 106:53–59
Takeda N, Manabe I, Shindo T, Iwata H, Iimuro S, Kagechika H (2006) Synthetic retinoid Am80 reduces scavenger receptor expression and atherosclerosis in mice by inhibiting IL-6. Arterioscler Thromb Vasc Biol 26:1177–1183
Davies NM, McLachlan AJ, Day RO, Williams KM (2000) Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet 38:225–242
Elrod HA, Yue P, Khuri FR, Sun SY (2009) Celecoxib antagonizes perifosine’s anticancer activity involving a cyclooxygenase-2-dependent mechanism. Mol Cancer Ther 8:2575–2585
Whitman SC, Ravisankar P, Elam H, Daugherty A (2000) Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E−/− mice. Am J Pathol 157:1819–1824
Podrez EA, Febbraio M, Sheibani N, Schmitt D, Silverstein RL, Hajjar DP, Cohen PA, Frazier WA, Hoff HF, Hazen SL (2000) Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest 105:1095–1108
Silverstein RL, Febbraio M (2000) CD36 and atherosclerosis. Curr Opin Lipidol 11:483–491
Nozaki S, Kashiwagi H, Yamashita S, Nakagawa T, Kostner B, Tomiyama Y, Nakata A, Ishigami M, Miyagawa J, Kameda-Takemura K (1995) Reduced uptake of oxidized low density lipoprotein in monocyte-derived macrophages from CD36-deficient subjects. J Clin Invest 96:1859–1865
Sugano R, Yamamura T, Harada-Shiba M, Miyake Y, Yamamoto A (2001) Uptake of oxidized low-density lipoprotein in a THP-1 cell line lacking scavenger receptor A. Atherosclerosis 158:351–357
Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL (2000) Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest 105:1049–1056
Kuchibhotla S, Vanegas D, Kennedy DJ, Guy E, Nimako G, Morton RE, Febbraio M (2008) Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res 78:185–196
Nicholson AC, Frieda S, Pearce A, Silverstein RL (1995) Oxidized LDL binds to CD36 on human monocyte-derived macrophages and transfected cell lines. Evidence implicating the lipid moiety of the lipoprotein as the binding site. Arterioscler Thromb Vasc Biol 15:269–275
Park YM, Febbraio M, Silverstein RL (2009) CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest 119:136–145
Cao RY, St Amand T, Gräbner R, Habenicht AJ, Funk CD (2009) Genetic and pharmacological inhibition of the 5-lipoxygenase/leukotriene pathway in atherosclerotic lesion development in ApoE deficient mice. Atherosclerosis 203:395–400
Funk CD, Cao RY, Zhao L, Habenicht AJ (2006) Is there a role for the macrophage 5-lipoxygenase pathway in aortic aneurysm development in apolipoprotein E-deficient mice? Ann NY Acad Sci 1085:151–160
Riccioni G, Back M, Capra V (2010) Leukotrienes and atherosclerosis. Curr Drug Targets 11:882–887
Qiu H, Gabrielsen A, Agardh HE, Wan M, Wetterholm A, Wong CH et al (2006) Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc Natl Acad Sci USA A103:8161–8166
Graff J, Skarke C, Klinkhardt U, Watzer B, Harder S, Seyberth H et al (2007) Effects of selective COX-2 inhibition on prostanoids and platelet physiology in young healthy volunteers. J Thromb Haemost 5:2376–2385
Sennlaub F, Valamanesh F, Vazquez-Tello A, El-Asrar AM, Checchin D, Brault S, Gobeil F, Beauchamp MH, Mwaikambo B, Courtois Y, Geboes K, Varma DR, Lachapelle P, Ong H, Behar-Cohen F, Chemtob S (2003) Cyclooxygenase-2 in human, experimental ischemic proliferative retinopathy. Circulation 108:198–204
Viñals M, Bermúdez I, Llaverias G, Alegret M, Sanchez RM, Vázquez-Carrera M, Laguna JC (2005) Aspirin increases CD36, SR-BI, and ABCA1 expression in human THP-1 macrophages. Cardiovasc Res 66:141–149
Chuang PC, Lin YJ, Wu MH, Wing LY, Shoji Y, Tsai SJ (2010) Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis. Am J Pathol 176:850–860
Bujold K, Rhainds D, Jossart C, Febbraio M, Marleau S, Ong H (2009) CD36-mediated cholesterol efflux is associated with PPARγ activation via a MAPK-dependent COX-2 pathway in macrophages. Cardiovasc Res 83:457–464
Reiss AB, Vagell ME (2006) PPARgamma activity in the vessel wall: anti-atherogenic properties. Curr Med Chem 13:3227–3238
Greaves DR, Gordon S (2009) The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J Lipid Res 50:S282–S286
Reiss AB, Wan DW, Anwar K, Merrill JT, Wirkowski PA, Shah N, Cronstein BN, Chan ES, Carsons SE (2009) Enhanced CD36 scavenger receptor expression in THP-1 human monocytes in the presence of lupus plasma: linking autoimmunity and atherosclerosis. Exp Biol Med 234:354–360
Acknowledgments
This work was supported by an Innovative Research Grant from the Arthritis Foundation and by a Winthrop University Hospital Pilot and Feasibility Grant.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1.
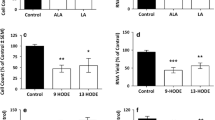
Dose-response curve for celecoxib effect on CD36, ScR-A1 and COX-2 expression in THP-1 monocytes. THP-1 monocytes were incubated with increasing concentrations of celecoxib for 18h. As demonstrated, increasing celecoxib (up to 50 μM) results in a decrease in COX-2 message and upregulation of CD36 message. However, ScR-A1 expression did not change significantly with increasing concentrations of celecoxib (n=3). **- P<0.01 vs. control cells in media alone, ***- P<0.001 vs. control cells. (PDF 14 kb)
Fig. 2.
Effect of COX-2 inhibition by celecoxib and cPLA-2 inhibition by AACOCF3 on CD36 and ScR-A1 mRNA expression in THP-1 monocytes. THP-1 monocytes were incubated for 18 hours under the following four conditions: 1) media alone, 2) IFN-γ [500u/ml], 3) AACOCF3 [25 μM], 4) celecoxib [25 μM]; 5) TGF-β (120 pg/ml). Following incubation, total RNA was isolated from cells exposed to each condition. RNA was reverse transcribed, CD36 and ScR-A1 messages were analyzed by QRT-PCR. The expressions of target genes were normalized to that of GAPDH. A. CD36 gene expression graphed as relative mRNA expression and compared with results obtained from untreated THP-1 monocytes. The data represent the mean and SEM of three independent experiments (n=3), **- P<0.01 vs. control cells in media alone, ## - P<0.01 vs. IFN – γ, AACOCF3, celecoxib. B. ScR-A1 gene expression graphed as relative mRNA expression and compared with results obtained from untreated THP-1 monocytes. The data represent the mean and SEM of three independent experiments (n=3), NS, ## - P<0.01 vs. control, IFN – γ, AACOCF3, celecoxib. (PDF 23 kb)
Fig. 3.
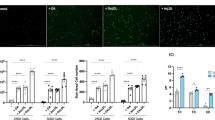
Effect of celecoxib and AACOCF3 on foam cell transformation in THP-1 macrophages under cholesterol loading conditions using oxidized LDL. THP-1 macrophages were treated with oxidized LDL (50 µg/ml, 48 hours). Cells were then exposed for 48 hours to the following five conditions: 1) untreated control; 2) IFN-γ [500 U/ml]; 3) celecoxib [25 µM]; 4) AACOCF3 [25 µM]; 5) TGF-β [120 pg/ml]. A. Cells were stained with Oil red O to detect foam cells. B. Graphic representation of percentage foam cells shows a significant increase in foam cell transformation with celecoxib, AACOCF3 or IFN-γ (n=4). ** - P<0.01 vs. control cells, *** - P<0.001 vs. control cells, # - P<0.05 vs. celecoxib, AACOCF3. (PDF 293 kb)
About this article
Cite this article
Anwar, K., Voloshyna, I., Littlefield, M.J. et al. COX-2 Inhibition and Inhibition of Cytosolic Phospholipase A2 Increase CD36 Expression and Foam Cell Formation in THP-1 Cells. Lipids 46, 131–142 (2011). https://doi.org/10.1007/s11745-010-3502-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-010-3502-4