Abstract
The mammalian liver, a key organ in lipid homeostasis, can accumulate increased amounts of lipids in certain physiological conditions including liver regeneration. Lipid droplets (LD), the lipid storage organelles in the cytoplasm, are composed of a core of neutral lipids (mainly triacylglycerols and cholesteryl esters) surrounded by a monolayer of phospholipids and cholesterol with associated proteins. It is recognized that LD lipid composition is cell- and environment-specific and enables LD to carry out specific functions, but few descriptive studies aiming to interpret such differences have been published. We characterized eight density fractions of LD isolated from quiescent (control) and regenerating liver after partial hepatectomy, and grouped populations according to their lipid composition. LD from quiescent liver resembled the cholesteryl ester storage LD found in steroidogenic tissues, whereas in the regenerating tissue they were similar to adipocyte LD. Specifically, there were large, light LD with increased triacylglycerol content, the hallmark of liver regeneration. The apparent volume of the dense LD was, however, lower than in the quiescent density-matched populations, concomitant with increased phosphatidylcholine and phosphatidylethanolamine and decreased neutral lipid content. Analysis of the lipid profile of LD populations from quiescent and regenerating tissue leads us to define four physiological LD phenotypes for rat liver.




Similar content being viewed by others
Abbreviations
- C:
-
Unesterified free cholesterol
- CE:
-
Cholesterol ester(s)
- LD:
-
Lipid droplet(s)
- PtdCho:
-
Phosphatidylcholine
- PtdEtn:
-
Phosphatidylethanolamine
- PH:
-
Partial hepatectomy
- PtdIns:
-
Phosphatidylinositol
- PL:
-
Phospholipid(s)
- PNSN:
-
Postnuclear supernatant
- PtdSer:
-
Phosphatidylserine
- CerPCho:
-
Sphingomyelin
- TAG:
-
Triacylglycerol
References
Fausto N (2000) Liver regeneration. J Hepatol 32:19–31
Michalopoulos GK (2007) Liver regeneration. J Cell Physiol 213:286–300
Michalopoulos GK (2009) Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol. doi:10.1016/j.biolcel.209.09.014
Taub R (2004) Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 5:836–847
Newberry EP, Kennedy SM, Xie Y, Luo J, Stanley SE, Semenkovich CF, Crooke RM, Graham MJ, Davidson NO (2008) Altered hepatic triglyceride content after partial hepatectomy without impaired liver regeneration in multiple murine genetic models. Hepatology 48:1097–1105
Shteyer E, Liao Y, Muglia LJ, Hruz PW, Rudnick DA (2004) Disruption of hepatic adipogenesis is associated with impaired liver regeneration in mice. Hepatology 40:1322–1332
DeAngelis RA, Markiewski MM, Taub R, Lambris JD (2005) A high-fat diet impairs liver regeneration in C57BL/6 mice through overexpression of the NF-kappaB inhibitor, IkappaBalpha. Hepatology 42:1148–1157
Fernandez MA, Albor C, Ingelmo-Torres M, Nixon SJ, Ferguson C, Kurzchalia T, Tebar F, Enrich C, Parton RG, Pol A (2006) Caveolin-1 is essential for liver regeneration. Science 313:1628–1632
Leclercq IA, Field J, Farrell GC (2003) Leptin-specific mechanisms for impaired liver regeneration in ob/ob mice after toxic injury. Gastroenterology 124:1451–1464
Yamauchi H, Uetsuka K, Okada T, Nakayama H, Doi K (2003) Impaired liver regeneration after partial hepatectomy in db/db mice. Exp Toxicol Pathol 54:281–286
Rinia HA, Burger KN, Bonn M, Muller M (2008) Quantitative label-free imaging of lipid composition and packing of individual cellular lipid droplets using multiplex CARS microscopy. Biophys J 95:4908–4914
Bartz R, Li WH, Venables B, Zehmer JK, Roth MR, Welti R, Anderson RG, Liu P, Chapman KD (2007) Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res 48:837–847
Hodges BD, Wu CC (2010) Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J Lipid Res 51:262–273
Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C (1997) Adipose differentiation-related protein is a ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38:2249–2263
Brasaemle DL, Dolios G, Shapiro L, Wang R (2004) Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3–L1 adipocytes. J Biol Chem 279:46835–46842
Cermelli S, Guo Y, Gross SP, Welte MA (2006) The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol 16:1783–1795
Dugail I, Hajduch E (2007) A new look at adipocyte lipid droplets: towards a role in the sensing of triacylglycerol stores? Cell Mol Life Sci 64:2452–2458
Fujimoto Y, Itabe H, Kinoshita T, Homma KJ, Onoduka J, Mori M, Yamaguchi S, Makita M, Higashi Y, Yamashita A, Takano T (2007) Involvement of long chain acyl-CoA synthetase in local synthesis of neutral lipids in cytoplasmic lipid droplets in human hepatocyte HuH7. J Lipid Res 48:1280–1292
Umlauf E, Csaszar E, Moertelmaier M, Schuetz GJ, Parton RG, Prohaska R (2004) Association of stomatin with lipid bodies. J Biol Chem 279:23699–23709
Welte MA (2007) Proteins under new management: lipid droplets deliver. Trends Cell Biol 17:363–369
Murphy DJ, Vance J (1999) Mechanisms of lipid-body formation. Trends Biochem Sci 24:109–115
Mendis-Handagama SM, Aten RF, Watkins PA, Scallen TJ, Berhman HR (1995) Peroxisomes and sterol carrier protein-2 in luteal cell steroidogenesis: a possible role in cholesterol transport from lipid droplets to mitochondria. Tissue Cell 27:483–490
Igal RA, Coleman RA (1998) Neutral lipid storage disease: a genetic disorder with abnormalities in the regulation of phospholipid metabolism. J Lipid Res 39:31–43
Waynforth HB, Flecknell PA (1992) Experimental and surgical technique in the rat. Academic Press, London, pp 241–245
Palacios L, Ochoa B, Jose Gomez-Lechon M, Vicente CJ, Fresnedo O (2006) Overexpression of SND p102, a rat homologue of p100 coactivator, promotes the secretion of lipoprotein phospholipids in primary hepatocytes. Biochim Biophys Acta 1761:698–708
García-Arcos I, Rueda Y, González-Kother P, Palacios L, Ochoa B, Fresnedo O (2010) Association of SND1 protein with low density lipid droplets in hepatic steatosis. J Physiol Biochem 66:73–83
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Ruiz JI, Ochoa B (1997) Quantification in the subnanomolar range of phospholipids and neutral lipids by monodimensional thin-layer chromatography and image analysis. J Lipid Res 38:1482–1489
Brasaemle DL (2006) A metabolic push to proliferate. Science 313:1581–1582
Grisham JW (1962) A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res 22:842–849
Farrell GC (2004) Probing Prometheus: fat fueling the fire? Hepatology 40:1252–1255
Ontko JA, Perrin LW, Horne LS (1986) Isolation of hepatocellular lipid droplets: the separation of distinct subpopulations. J Lipid Res 27:1097–1103
Blaner WS, O’Byrne SM, Wongsiriroj N, Kluwe J, D’Ambrosio DM, Jiang H, Schwabe RF, Hillman EM, Piantedosi R, Libien J (2008) Hepatic stellate cell lipid droplets: a specialized lipid droplet for retinoid storage. Biochim Biophys Acta 1791:408–418
Murphy DJ (2001) The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40:325–438
Jump DB, Clarke SD (1999) Regulation of gene expression by dietary fat. Annu Rev Nutr 19:63–90
Fuki IV, Preobrazhensky SN, Misharin AY, Bushmakina NG, Menschikov GB, Repin VS, Karpov RS (1989) Effect of cell cholesterol content on apolipoprotein B secretion and LDL receptor activity in the human hepatoma cell line, HepG2. Biochim Biophys Acta 1001:235–238
Isusi E, Aspichueta P, Liza M, Hernandez ML, Diaz C, Hernandez G, Martinez MJ, Ochoa B (2000) Short- and long-term effects of atorvastatin, lovastatin and simvastatin on the cellular metabolism of cholesteryl esters and VLDL secretion in rat hepatocytes. Atherosclerosis 153:283–294
Temel RE, Hou L, Rudel LL, Shelness GS (2007) ACAT2 stimulates cholesteryl ester secretion in apoB-containing lipoproteins. J Lipid Res 48:1618–1627
Field FJ, Mathur SN, LaBrecque DR (1985) Cholesterol metabolism in regenerating liver of the rat. Am J Physiol 249:G679–G684
Lo Sasso G, Celli N, Caboni M, Murzilli S, Salvatore L, Morgano A, Vacca M, Pagliani T, Parini P, Moschetta A (2010) Down-regulation of the LXR transcriptome provides the requisite cholesterol levels to proliferating hepatocytes. Hepatology 51:1334–1344
Delahunty TJ, Rubinstein D (1970) Accumulation and release of triglycerides by rat liver following partial hepatectomy. J Lipid Res 11:536–543
Houweling M, Tijburg LB, Vaartjes WJ, Van Golde LM (1992) Phosphatidylethanolamine metabolism in rat liver after partial hepatectomy. Control of biosynthesis of phosphatidylethanolamine by the availability of ethanolamine. Biochem J 283(Pt 1):55–61
Tijburg LB, Nyathi CB, Meijer GW, Geelen MJ (1991) Biosynthesis and secretion of triacylglycerol in rat liver after partial hepatectomy. Biochem J 277(Pt 3):723–728
Vance DE, Vance JE (2008) Biochemistry of lipids, lipoproteins and membranes. Elsevier, Amsterdam
Acknowledgments
Research was supported by grants from the Spanish National Ministry for Education and Science (SAF2007/60211) and the Basque Government (Saiotek program and IT-325-07). I.G-A. was recipient of a research training fellowship from the Basque Government.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
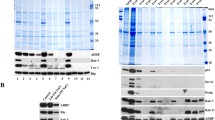
Supplementary Fig. 1. Identification of isolated lipid droplet populations in quiescent and regenerating rat liver.
a. Postnuclear supernatant (PNSN) from quiescent and regenerating rat liver (0.32 g) was fractionated on a sucrose gradient as described in Material and Methods (depicted in Fig. 2 of the manuscript). Eight fractions, harvested from the top of the gradient, and microsomes as a negative control were probed with antibodies against the lipid droplet marker adipophilin (ADRP), the Golgi marker GM-130, and the endoplasmic reticulum marker calregulin. It was confirmed that fractions 1–8 were adipophilin(+) lipid droplets, without visible contamination. (TIFF 811 kb)
About this article
Cite this article
García-Arcos, I., González-Kother, P., Aspichueta, P. et al. Lipid Analysis Reveals Quiescent and Regenerating Liver-Specific Populations of Lipid Droplets. Lipids 45, 1101–1108 (2010). https://doi.org/10.1007/s11745-010-3492-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-010-3492-2