Abstract
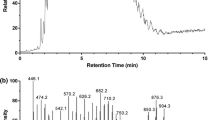
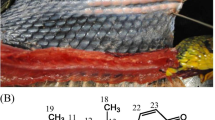
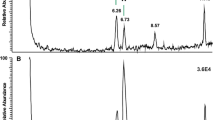
Unusual fatty acids with 24, 26, and 28 carbon atoms were found in triacylglycerols (TAGs) isolated from fat body tissue of bumblebee Bombus pratorum. The most abundant one was (Z,Z)-9,19-hexacosadienoic acid. Its structure was determined by mass spectrometry after derivatization with dimethyl disulfide and by infrared spectroscopy. ECL (equivalent chain length) values of its methyl ester were determined on both DB-1 and DB-WAX capillary columns. (Z,Z)-9,19-Hexacosadienoic acid is quite rare in nature. So far it has been identified only in marine sponges, and this work is the first evidence of its occurrence in a terrestrial organism. HPLC/MS analysis of the bumblebee TAGs showed that (Z,Z)-9,19-hexacosadienoic acid is present in one third of all TAG molecular species. As it was found in all sn-TAG positions, it is likely that (Z,Z)-9,19-hexacosadienoic acid is transported to tissues. Interestingly, labial gland secretion of B. pratorum was found to contain (Z,Z)-7,17-pentacosadiene, a hydrocarbon with markedly similar double bond positions and geometry. Possible biosynthetic relationships between these two compounds are discussed.





Similar content being viewed by others
References
Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA (2001) Fat metabolism in insects. Annu Rev Nutr 21:23–46. doi:10.1146/annurev.nutr.21.1.23
Stanley-Samuelson DW, Nelson DR (ed) (1993) Insect lipids: chemistry, biochemistry and biology. University of Nebraska Press, Lincoln, NE
Luxová A, Valterová I, Stránský K, Hovorka O, Svatoš A (2003) Biosynthetic studies on marking pheromones of bumblebee males. Chemoecology 13:81–87. doi:10.1007/s00049-003-0230-8
Bashan M, Cakmak O (2005) Changes in composition of phospholipid and triacylglycerol fatty acids prepared from prediapausing and diapausing individuals of Dolycoris baccarum and Piezodorus lituratus (Heteroptera: Pentatomidae). Ann Entomol Soc Am 98:575–579. doi:10.1603/0013-8746(2005)098[0575:CICOPA]2.0.CO;2
Hoback WW, Rana RL, Stanley DW (1999) Fatty acid compositions of phospholipids and triacylglycerols of selected tissues, and fatty acid biosynthesis in adult periodical cicadas, Magicicada septendecim. Comp Biochem Physiol A Mol Integr Physiol 122:355–362. doi:10.1016/S1095-6433(99)00018-5
Canavoso LE, Bertello LE, de Lederkremer RM, Rubiolo ER (1998) Effect of fasting on the composition of the fat body lipid of Dipetalogaster maximus, Triatoma infestans and Panstrongylus megistus (Hemiptera: Reduviidae). J Comp Physiol B 168:549–554. doi:10.1007/s003600050176
Cvačka J, Hovorka O, Jiroš P, Kindl J, Stránský K, Valterová I (2006) Analysis of triacylglycerols in fat body of bumblebees by chromatographic methods. J Chromatogr A 1101:226–237. doi:10.1016/j.chroma.2005.10.001
Stránský K, Jursík T (1996) Simple quantitative transesterification of lipids. 1. Introduction. Fett/Lipid 98:65–71. doi:10.1002/lipi.19960980206
Stránský K, Jursík T, Vítek A, Skořepa J (1992) An improved method of characterizing fatty-acids by equivalent chain-length values. J High Res Chromatogr 15:730–740. doi:10.1002/jhrc.1240151107
Holčapek M, Lísa M, Jandera P, Kabátová N (2005) Quantitation of triacylglycerols in plant oils using HPLC with APCI-MS, evaporative light-scattering, and UV detection. J Sep Sci 28:1315–1333. doi:10.1002/jssc.200500088
Cvačka J, Krafková E, Jiroš P, Valterová I (2006) Computer-assisted interpretation of atmospheric pressure chemical ionization mass spectra of triacylglycerols. Rapid Commun Mass Spectrom 20:3586–3594. doi:10.1002/rcm.2770
Dunkelblum E, Tan SH, Sikl PJ (1985) Double-bond location in monounsaturated fatty acids by dimethyl disulfide derivatization and mass spectrometry: application to analysis of fatty acids in pheromone glands of four Lepidoptera. J Chem Ecol 11:265–277. doi:10.1007/BF01411414
Vincenti M, Guglielmetti G, Cassani G, Tonini C (1987) Determination of double bond position in diunsaturated compounds by mass spectrometry of dimethyl disulfide derivatives. Anal Chem 59:694–699. doi:10.1021/ac00132a003
Stránský K, Jursík T, Vítek A (1997) Standard equivalent chain length values of monoenic and polyenic (methylene interrupted) fatty acids. J High Res Chromatogr 20:143–158. doi:10.1002/jhrc.1240200305
Hamilton RJ, Cast J (1999) Spectral properties of lipids (the chemistry and technology of oils and fats). Sheffield Academic Press Ltd., Sheffield, UK
Attygalle AB, Svatoš A, Wilcox C, Voerman S (1994) Gas-phase infrared spectroscopy for determination of double bond configuration of monounsaturated compounds. Anal Chem 66:1696–1703. doi:10.1021/ac00082a016
Christie WW (2003) Lipid analysis: isolation, separation, identification and structural analysis of lipids. The Oily Press, Bridgwater, UK
Morales RW, Litchfield C (1977) Incorporation of 1–14C-acetate into C26 fatty acids of the marine sponge Microciona prolifera. Lipids 12:570–576. doi:10.1007/BF02533383
Christie WW, Brechany EY, Stefanov K, Popov S (1992) The fatty acids of the sponge Dysidea fragilis from the Black Sea. Lipids 27:640–644. doi:10.1007/BF02536125
Rod’kina SA, Latyshev NA, Imbs AB (2003) Fatty acids from the Sea of Japan sponge Halichondria panicea. Russ J Bioorg Chem (Translation of Bioorganicheskaya Khimiya) 29:382–386. doi:10.1023/A:1024957403078
Christie WW, Brechany EY, Marekov IN, Stefanov KL, Andreev SN (1994) The fatty acids of the sponge Hymeniacidon sanguinea from the Black Sea. Comp Biochem Physiol B Biochem Mol Biol 109B:245–252. doi:10.1016/0305-0491(94)90008-6
Nechev J, Christie WW, Robaina R, de Diego F, Popov S, Stefanov K (2004) Chemical composition of the sponge Hymeniacidon sanguinea from the Canary Islands. Comp Biochem Physiol A Mol Integr Physiol 137:365–374. doi:10.1016/j.cbpb.2003.10.016
Lam WK, Hahn S, Ayanoglu E, Djerassi C (1989) Phospholipid studies of marine organisms. 22. Structure and biosynthesis of a novel brominated fatty acid from a hymeniacidonid sponge. J Org Chem 54:3428–3432. doi:10.1021/jo00275a032
Carballeira NM, Reyes ED, Shalabi F (1993) Identification of novel iso/anteiso nonacosadienoic acids from the phospholipids of the sponges Chondrosia remiformis and Myrmekioderma styx. J Nat Prod 56:1850–1855. doi:10.1021/np50100a032
Carballeira NM, Pagan M, Rodriguez AD (1998) Identification and total synthesis of novel fatty acids from the caribbean sponge Calyx podatypa. J Nat Prod 61:1049–1052. doi:10.1021/np9801413
Rod’kina SA (2005) Fatty acids from the sponge Tedania dirhaphis. Chem Nat Comp 41:289–292. doi:10.1007/s10600-005-0131-x
Dembitsky VM, Řezanka T, Kashin AG (1993) Comparative study of the endemic freshwater fauna of Lake Baikal-II. Unusual lipid composition of two sponge species Baicalospongia bacillifera and Baicalospongia intermedia (family Lubomirskiidae, class Demospongiae). Comp Biochem Physiol B Biochem Mol Biol 106B:825–831. doi:10.1016/0305-0491(93)90037-6
Dembitsky VM, Řezanka T, Kashin AG (1994) Comparative study of the endemic freshwater fauna of Lake Baikal. VI. Unusual fatty acid and lipid composition of the endemic sponge Lubomirskia baicalensis and its amphipod crustacean parasite Brandtia (Spinacanthus) parasitica. Comp Biochem Physiol B Biochem Mol Biol 109B:415–426. doi:10.1016/0305-0491(94)90024-8
Shantha NC, Ackman RG (1991) Fish oil tetracosenoic acid isomers and GLC analyses of polyunsaturated fatty acids. Lipids 26:237–239. doi:10.1007/BF02543978
Takayama K, Qureshi N, Schnoes HK (1978) Isolation and characterization of the monounsaturated long chain fatty acids of Mycobacterium tuberculosis. Lipids 13:575–579. doi:10.1007/BF02535818
Nichols PD, Mayberry WR, Antworth CP, White DC (1985) Determination of monounsaturated double-bond position and geometry in the cellular fatty acids of the pathogenic bacterium Francisella tularensis. J Clin Microbiol 21:738–740
Mottram HR, Evershed RP (1996) Structure analysis of triacylglycerol positional isomers using atmospheric pressure chemical ionisation mass spectrometry. Tetrahedron Lett 37:8593–8596. doi:10.1016/0040-4039(96)01964-8
Kullenberg B, Bergström G, Ställberg-Stenhagen S (1970) Volatile components of the cephalic marking secretion of male bumblebees. Acta Chem Scand 24:1481–1483
Lanne BS, Bergström G, Wassgren A-B, Törnbäck B (1987) Biogenetic pattern of straight chain marking compounds in male bumble bees. Comp Biochem Physiol 88B:631–636. doi:10.1016/0305-0491(87)90355-5
Svenson BG, Bergström G (1977) Volatile marking secretions from the labial gland of North European Pyrobombus D. T. males (Hymenoptera, Apidae). Insectes Soc 24:213–224. doi:10.1007/BF02227172
Ponchau O, Terzo M, Aytekin M, Valterová I, Iserbyt S, Michez D, Rasmont P (2006) The geographical variability of the secretions of the cephalic labial glands of Bombus pratorum L. males. In: XVth IUSSI Congr, Washington, DC, 30 July–5 Aug 2006, p 526
Cahlíková L (2008) Isolation and identification of compounds influencing the behavior of Hymenoptera. PhD Thesis, Institute of Chemical Technology, Prague, Czech Republic
Tillman JA, Seybold SJ, Jurenka RA, Blomquist GJ (1999) Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem Mol Biol 29:481–514. doi:10.1016/S0965-1748(99)00016-8
Acknowledgments
The financial support from the Grant Agency of the Academy of Sciences of the Czech Republic (A4055403), by the Ministry of Education of the Czech Republic (2B06007), and by the Academy of Sciences of the Czech Republic (research project No. Z40550506) is acknowledged with appreciation.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Cvačka, J., Kofroňová, E., Vašíčková, S. et al. Unusual Fatty Acids in the Fat Body of the Early Nesting Bumblebee, Bombus pratorum . Lipids 43, 441–450 (2008). https://doi.org/10.1007/s11745-008-3174-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-008-3174-5