Abstract
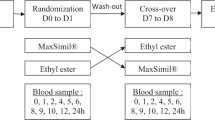
To investigate octacosanol (OC) metabolism in humans and its influence on cholesterol metabolism, two studies were conducted. In the first study ten healthy women received daily 30 mg OC for a period of 4 weeks. Blood and feces samples were collected at baseline and after the intervention. Serum concentrations of total cholesterol, LDL cholesterol, and HDL cholesterol were not altered following OC administration. Concentrations of excreted cholesterol end products decreased with the intervention (neutral sterols: 24.6 ± 9.7 mg/g vs. 20.3 ± 7.5 mg/g dry matter, P < 0.05; bile acids: 6.47 ± 3.89 mg/g vs. 4.03 ± 2.26 mg/g dry matter, P < 0.05). OC was not detected in serum samples, but the fecal OC concentration increased after the intervention period (11 ± 7 μg/g vs. 817 ± 179 μg/g dry matter, P < 0.05). In the second kinetic study on three participants, OC was identified in serums after oral application of 50 mg OC within 8 h. The decrease in the concentration of fecal cholesterol end products may underline a systemic effect of OC on cholesterol metabolism, even though the serum cholesterol levels were not influenced.


Similar content being viewed by others
Abbreviations
- BA:
-
Bile acid
- FA:
-
Fatty acid
- FAME:
-
Fatty acid methyl ester
- NS:
-
Neutral sterol
- OC:
-
Octacosanol
- TC:
-
Total cholesterol
References
Nies LK, Cymbala AA, Kasten SL, Lamprecht DG, Olson KL (2006) Complementary and alternative therapies for the management of dyslipidemia. Ann Pharmacother 40:1984–1992
Varady KA, Wang Y, Jones P (2003) Role of policosanols in the prevention and treatment of cardiovascular disease. Nutr Rev 61:376–383
Janikula M (2002) Policosanol: a new treatment for cardiovascular disease? Altern Med Rev 7:203–217
Crespo N, Illnait J, Más R, Fernández L, Fernández J, Castaño G (1999) Comparative study of the efficacy and tolerability of policosanol and lovastatin in patients with hypercholesterolemia and noninsulin dependent diabetes mellitus. Int J Clin Pharmacol Res 19:117–127
Fernández JC, Más R, Castaño G, Menéndez R, Amor A, González RM, Alvarez E (2001) Comparison of the efficacy, safety and tolerability of policosanol versus fluvastatin in elderly hypercholesterolaemic women. Clin Drug Investig 21:103–113
Castaño G, Menéndez R, Más R, Amor A, Fernández JL, González RL, Lezcay M, Alvarez E (2002) Effects of policosanol and lovastatin on lipid profile and lipid peroxidation in patients with dyslipidemia associated with type 2 diabetes mellitus. Int J Clin Pharmacol Res 22:89–100
Cubeddu LX, Cubeddu RJ, Heimowitz T, Restrepo B, Lamas G, Weinberg GB (2006) Comparative lipid-lowering effects of policosanol and atorvastatin: a randomized, parallel, double-blind, placebo-controlled trial. Am Heart J 152:982.e1–982.e5
Greyling A, De Witt C, Oosthuizen W, Jerling JC (2006) Effects of a policosanol supplement on serum lipid concentrations in hypercholesterolemic subjects. Br J Nutr 95:968–975
Lin Y, Rudrum M, Van der Wielen RPJ, Trautwein EA, McNeill G, Sierksma A, Meijer GW (2004) Wheat germ policosanol failed to lower plasma cholesterol in subjects with normal to mildly elevated cholesterol concentrations. Metabolism 53:1309–1314
Berthold HK, Unverdorben S, Degenhardt R, Bulitta M, Gouni-Berthold I (2006) Effect of policosanol on lipid levels among patients with hypercholesterolemia or combined hyperlipidemia. A randomized controlled trial. J Am Med Assoc 295:2262–2269
Rizzo WB, Craft DA, Dammann AL, Phillips MW (1987) Fatty alcohol metabolism in cultured human fibroblasts. Evidence for a fatty alcohol cycle. J Biol Chem 262:17412–17419
Menéndez R, Marrero D, Más R, Fernández I, González L, González RM (2005) In vitro and in vivo study of octacosanol metabolism. Arch Med Res 36:113–119
Más R (2000) Policosanol. Hypolipidemic antioxidant treatment of atherosclerosis. Drugs Future 25:569–586
Menéndez R, Más R, Amor AM, Rodeiro I, González RM, Alfonso JL (2001) Inhibition of cholesterol biosynthesis in cultured fibroblasts by D003, a mixture of very long chain saturated fatty acids. Pharmacol Res 44:299–304
Castaño G, Más R, Fernández L, López E, Gutiérrez JA, Illnait J, Fernández JC, Gámez R, Alvarez E (2002) Assessment of the effects of D-003, a new antiplatelet and lipid-lowering compound, in healthy volunteers. A phase I clinical study. Drugs R D 3:337–348
Castaño G, Más R, Fernández L, Illnait J, López E, Gámez R, Mendoza S, Fernández J, Mesa M (2003) Effects of D-003 on the lipid profile of patients with type II hypercholesterolaemia. A phase II clinical study. Clin Drug Investig 23:789–802
Menéndez R, Más R, Pérez J, González RM, Jiménez S (2004) Oral administration of D-003, a mixture of very long chain fatty acids prevents casein-induced endogenous hypercholesterolemia in rabbits. Can J Physiol Pharmacol 82:22–29
Gouni-Berthold I, Berthold HK (2002) Policosanol: clinical pharmacology and therapeutic significance of a new lipid-lowering agent. Am Heart J 143:356–365
Singh DK, Li L, Porter TD (2006) Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of AMP-kinase. J Pharmacol Exp Ther 318:1020–1026
Menéndez R, Fernández SI, Del Rio A, González RM, Fraga V, Amor AM, Más RM (1994) Policosanol inhibits cholesterol biosynthesis and enhances low density lipoprotein processing in cultured human fibroblasts. Biol Res 27:199–203
Menéndez R, Arruzazabala L, Más R, Del Rio A, Amor AM, González RM, Carbajal D, Fraga V, Molina V, Illnait J (1997) Cholesterol-lowering effect of policosanol on rabbits with hypercholesterolemia induced by a wheat starch-casein diet. Br J Nutr 77:923–932
Menéndez R, Amor AM, Rodeiro I, González RM, González PC, Alfonso JL, Más R (2001) Policosanol modulates HMG-CoA reductase activity in cultured fibroblasts. Arch Med Res 32:8–12
Menéndez R, Sotolongo V, Fraga V, Amor AM, González R, Del Rio A, Jiménez S, Pérez N, Más R (1996) Plasma levels and total radioactivity excretion in healthy volunteers after oral 3H-octacosanol administration. Rev CENIC Cien Biol 27:32–35
Kabir Y, Kimura S (1993) Biodistribution and metabolism of orally administered octacosanol in rats. Ann Nutr Metab 37:33–38
Kabir Y, Kimura S (1994) Distribution of radioactive octacosanol in response to exercise in rats. Nahrung 38:373–377
Kabir Y, Kimura S (1995) Tissue distribution of (8-14C)-octacosanol in liver and muscle of rats after serial administration. Ann Nutr Metab 39:279–284
Marinangeli CPF, Kassis AN, Jain D, Ebine N, Cunnane SC, Jones PJH (2007) Comparison of composition and absorption of sugarcane policosanol. Br J Nutr 97:381–388
Ditscheid B, Keller S, Jahreis G (2005) Cholesterol metabolism is affected by calcium phosphate supplementation in humans. J Nutr 135:1678–1682
Keller S, Jahreis G (2004) Determination of underivatised sterols and bile acid trimethyl silyl ether methyl esters by gas chromatography-mass spectrometry-single ion monitoring in faeces. J Chromatogr B Analyt Technol Biomed Life Sci 813:199–207
Kraft J, Hanske L, Möckel P, Zimmermann S, Härtl A, Kramer JKG, Jahreis G (2006) The conversion efficiency of trans-11 and trans-12 18:1 by Δ9-desaturation differs in rats. J Nutr 136:1209–1214
Wilkins TD, Hackman AS (1974) Two patterns of neutral steroid conversion in the feces of normal north Americans. Cancer Res 34:2250–2254
Pepping J (2003) Policosanol. Am J Health Syst Pharm 60:1112–1115
Hernández F, Illnait J, Más R, Castaño G, Fernádez L, González M, Cordovi N, Fernandez JC (1992) Effect of policosanol on serum lipids and lipoproteins in healthy volunteers. Curr Ther Res Clin Exp 51:568–575
Shefer S, Hauser S, Lapar V, Mosbach EH (1973) Regulatory effects of sterols and bile acids on hepatic 3-hydroxy-3-methylglutaryl CoA reductase and cholesterol 7α-hydroxylase in the rat. J Lipid Res 14:573–580
Wang YW, Jones PJH, Pischel I, Fairow C (2003) Effects of policosanols and phytosterols on lipid levels and cholesterol biosynthesis in hamsters. Lipids 38:165–170
Hargrove JL, Greenspan P, Hartle DK (2004) Nutritional significance and metabolism of very long chain fatty alcohols and acids from dietary waxes. Exp Biol Med 229:215–226
Ng CH, Leung KY, Huang Y, Chen ZY (2005) Policosanol has no antioxidant activity in human low-density lipoprotein but increases excretion of bile acids in hamsters. J Agric Food Chem 53:6289–6293
Author information
Authors and Affiliations
Corresponding author
Additional information
Dual first authorship. S. Keller and F. Gimmler having contributed equally to the basic science presented.
About this article
Cite this article
Keller, S., Gimmler, F. & Jahreis, G. Octacosanol Administration to Humans Decreases Neutral Sterol and Bile Acid Concentration in Feces. Lipids 43, 109–115 (2008). https://doi.org/10.1007/s11745-007-3127-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-007-3127-4