Abstract
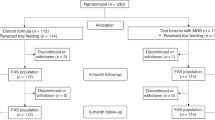
Healthy term infants (n = 244) were randomized to receive: (1) control, soy-based formula without supplementation or (2) docosahexaenoic acid−arachidonic acid (DHA + ARA), soy-based formula supplemented with at least 17 mg DHA/100 kcal (from algal oil) and 34 mg ARA/100 kcal (from fungal oil) in a double-blind, parallel group trial to evaluate safety, benefits, and growth from 14 to 120 days of age. Anthropometric measurements were taken at 14, 30, 60, 90, and 120 days of age and 24-h dietary and tolerance recall were recorded at 30, 60, 90, and 120 days of age. Adverse events were recorded throughout the study. Blood samples were drawn from subsets of 25 infants in each group. Capillary column gas chromatography was used to analyze the percentages of fatty acids in red blood cell (RBC) lipids and plasma phospholipids. Compared with the control group, percentages of fatty acids such as DHA and ARA in total RBC and plasma phospholipids were significantly higher in infants in the DHA + ARA group at 120 days of age (P < 0.001). Growth rates did not differ significantly between feeding groups at any assessed time point. Supplementation did not affect the tolerance of formula or the incidence of adverse events. Feeding healthy term infants soy-based formula supplemented with DHA and ARA from single cell oil sources at concentrations similar to human milk significantly increased circulating levels of DHA and ARA when compared with the control group. Both formulas supported normal growth and were well tolerated.

Similar content being viewed by others
References
Martinez M (1992) Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr 120:S129–S138
Innis SM (2003) Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr 143:S1–S8
Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA (1993) Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci USA 90:1073–1077
Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA (1994) Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr 60:189–194
Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF (2001) The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res 40:1–94
Innis SM, Gilley J, Werker J (2001) Are human milk long-chain polyunsaturated fatty acids related to visual and neural development in breast-fed term infants? J Pediatr 139:532–538
Birch EE, Garfield S, Castaneda Y, Hughbanks-Wheaton D, Uauy R, Hoffman D (2007) Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev 83:279–284
Innis SM (1992) Human milk and formula fatty acids. J Pediatr 120:S56–S61
Koletzko B, Thiel I, Abiodun PO (1992) The fatty acid composition of human milk in Europe and Africa. J Pediatr 120:S62–S70
Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM (2007) Docosahexaenoic and arachidonic acid concentrations in human breast milks worldwide. Am J Clin Nutr 85:1457–1464
Clandinin MT, Van Aerde JE, Parrott A, Field CJ, Euler AR, Lien EL (1997) Assessment of the efficacious dose of arachidonic and docosahexaenoic acids in preterm infant formulas: fatty acid composition of erythrocyte membrane lipids. Pediatr Res 42:819–825
Hoffman DR, Birch EE, Birch DG, Uauy R, Castaneda YS, Lapus MG, et al. (2000) Impact of early dietary intake and blood lipid composition of long-chain polyunsaturated fatty acids on later visual development. J Pediatr Gastroenterol Nutr 31:540–553
Hoffman DR, Wheaton DK, James KJ, Tuazon M, Diersen-Schade DA, Harris CL, et al. (2006) Docosahexaenoic acid in red blood cells of term infants receiving two levels of long-chain polyunsaturated fatty acids. J Pediatr Gastroenterol Nutr 42:287–292
Birch EE, Hoffman DR, Uauy R, Birch DG, Prestidge C (1998) Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr Res 44:201–209
Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG (2000) A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol 42:174–181
Birch EE, Hoffman DR, Castaneda YS, Fawcett SL, Birch DG, Uauy RD (2002) A randomized controlled trial of long-chain polyunsaturated fatty acid supplementation of formula in term infants after weaning at 6 wk of age. Am J Clin Nutr 75:570–580
Hoffman DR, Birch EE, Castaneda YS, Fawcett SL, Wheaton DH, Birch DG, et al. (2003) Visual function in breast-fed term infants weaned to formula with or without long-chain polyunsaturates at 4 to 6 months: a randomized clinical trial. J Pediatr 142:669–677
Morale SE, Hoffman DR, Castaneda YS, Wheaton DH, Burns RA, Birch EE (2005) Duration of long-chain polyunsaturated fatty acids availability in the diet and visual acuity. Early Hum Dev 81:197–203
Innis SM, Adamkin DH, Hall RT, Kalhan SC, Lair C, Lim M, et al. (2002) Docosahexaenoic acid and arachidonic acid enhance growth with no adverse effects in preterm infants fed formula. J Pediatr 140:547–554
Clandinin MT, Van Aerde JE, Merkel KL, Harris CL, Springer MA, Hansen JW, et al. (2005) Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J Pediatr 146:461–8
AAP (1998) Soy protein-based formulas: recommendations for use in infant feeding. Pediatr 101:148–153
Merritt R, Jenks B (2004) Safety of soy-based infant formulas containing isoflavones: the clinical evidence. J Nutr 134:1220S–1224S
Severity scoring of atopic dermatitis: the SCORAD index (1993) Consensus report of the European task force on atopic dermatitis. Dermatology 186:23–31
Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. (2002) Centers for disease control and prevention 2000 growth charts for the United States: improvements to the 1977 national center for health statistics version. Pediatrics 109:45–60
American Academy of Pediatrics (1988) Clinical testing of infant formulas with respect to nutritional suitability for term infants. Report to the FDA. Committee on Nutrition. AAP Elk Grove Village, IL
Fomon S, Ziegler E (1992) Isolated soy protein in infant feeding. In: Steinke F, Waggle D, Volgarev M (eds) New protein foods in human health: nutrition, prevention, and therapy. CRC, Boca Raton
Churella HR, Borschel MW, Thomas MR, Breen M, Jacobs J (1994) Growth and protein status of term infants fed soy protein formulas differing in protein content. J Am Coll Nutr 13:262–267
Lasekan JB, Ostrom KM, Jacobs JR, Blatter MM, Ndife LI, Gooch WM 3rd, et al. (1999) Growth of newborn, term infants fed soy formulas for 1 year. Clin Pediatr (Phila) 38:563–571
Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, et al. (2001) Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 286:807–814
Seppo L, Korpela R, Lonnerdal B, Metsaniitty L, Juntunen-Backman K, Klemola T, et al. (2005) A follow-up study of nutrient intake, nutritional status, and growth in infants with cow milk allergy fed either a soy formula or an extensively hydrolyzed whey formula. Am J Clin Nutr 82:140–145
Osborn DA, Sinn J (2006) Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev 4:CD003741
Auestad N, Montalto MB, Hall RT, Fitzgerald KM, Wheeler RE, Connor WE, et al. (1997) Visual acuity, erythrocyte fatty acid composition, and growth in term infants fed formulas with long chain polyunsaturated fatty acids for one year. Ross pediatric lipid study. Pediatr Res 41:1–10
Auestad N, Halter R, Hall RT, Blatter M, Bogle ML, Burks W, et al. (2001) Growth and development in term infants fed long-chain polyunsaturated fatty acids: a double-masked, randomized, parallel, prospective, multivariate study. Pediatrics 108:372–381
Birch EE, Castaneda YS, Wheaton DH, Birch DG, Uauy RD, Hoffman DR (2005) Visual maturation of term infants fed long-chain polyunsaturated fatty acid-supplemented or control formula for 12 mo. Am J Clin Nutr 81:871–879
Acknowledgments
This study was supported by a grant from Mead Johnson & Company. The authors wish to thank study investigators and their staff for their cooperation, including Barbara Alexander, M.D. (Pediatric Associates, Hollywood, FL), Dean Antonson, M.D. (The Center for Human Nutrition, Omaha, NE), Janet Barnes, M.D. (New Orleans, LA), Azzam Baker, M.D. (Riverside Pediatric Group, Secaucus, NJ), Wesley Burks, M.D. (Arkansas Children’s Hospital, Little Rock, AR), William M. Crecelius, M.D. (Welborn Clinic, Evansville, IN); James Hubbard, M.D. (Medical Associates Clinic, Dubuque, IA); William H. Johnson, Jr., M.D. (Birmingham, AL), Barry Kroll, M.D. (Margiotti & Kroll Pediatrics, Newtown, PA); Matthew Sadof, M.D. (Baystate Pediatric Group, Springfield, MA); Charles Sheaffer, M.D. (Chapel Hill, NC); and Eric Slosberg, M.D. (ProMed Pediatrics, Kalamazoo, MI). The participation of parents and infants in this study is gratefully acknowledged. We also acknowledge valuable contributions from Julia Boettcher, R.D., M.S. and Suzanne I. Stolz in the execution and completion of this study. We are grateful to Dianna K.H. Wheaton, K.J. James, and L.E. Wiedemann for fatty acid analysis conducted at the Retina Foundation. Additionally, we would like to acknowledge Jennifer Wampler, Ph.D. for her contributions to the manuscript development.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Hoffman, D., Ziegler, E., Mitmesser, S.H. et al. Soy-Based Infant Formula Supplemented with DHA and ARA Supports Growth and Increases Circulating Levels of these Fatty Acids in Infants. Lipids 43, 29–35 (2008). https://doi.org/10.1007/s11745-007-3116-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11745-007-3116-7