Abstract
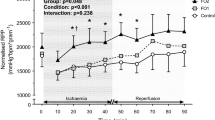
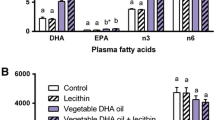
Fish consumption is associated with reduced cardiovascular mortality, and elevated myocardial long-chain n−3 polyunsaturated FA (PUFA) content is implicated in this cardioprotection. This study examined the dose and time responses for incorporation of n−3 PUFA into cellular membranes in rats fed fish oil (FO)-containing diets. For the time course study, rats were fed a 10% FO diet for periods ranging from 0 to 42 d, after which myocardial and erythrocyte membrane fatty acid composition was determined. For the dose response study, rats (n=3) were fed 0, 1.25, 2.5, 5, or 10% FO for 4 wk, with myocardial, erythrocyte, and skeletal muscle membrane FA determined. Myocardial DHA (22∶6n−3) levels doubled in 2 d, stabilizing at levels ≈200% higher than control after 28 d feeding with 10% FO. By comparison, DHA levels doubled after 4 wk of 1.25% FO feeding. In myocardium and skeletal muscle, EPA (20∶5n−3) levels remained low, but in erythrocytes EPA levels reached 50% of DHA levels. The n−3 PUFA were incorporated at the expense of n−6 PUFA in myocardium and skeletal muscle, whereas erythrocytes maintained arachidonic acid levels, and total n−3 PUFA incorporation was lower. This study shows that low doses of FO produce marked changes in myocardial DHA levels; maximal incorporation takes up to 28 d to occur; and while erythrocytes are a good indicator of tissue n−3 incorporation in stable diets, they vary greatly in their time course and pattern of incorporation.
Similar content being viewed by others
Abbreviations
- EPA:
-
eicosapentaenoic acid
- DHA:
-
docosahexaenoic acid
- FO:
-
fish oil
- i.p.:
-
intraperitoneal
- OO:
-
olive oil
- PUFA:
-
polyunsaturated fatty acid(s)
References
Dyerberg J. and Bang H. O. (1982) A hypothesis on the development of acute myocardial infarction in Greenlanders. Scand. J. Clin. Lab. Invest. Suppl. 161, 7–13.
Oomen C. M., Feskens E. J., Rasanen L., Fidanza F., Nissinen A. M., Menotti A., Kok F. J. and Kromhout D. (2000) Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. Am. J. Epidemiol. 151, 999–1006.
Albert C. M., Campos H., Stampfer M. J., Ridker P. M., Manson J. E., Willett W. C., and Ma J. (2002) Blood levels of long-chain n−3 FA and the risk of sudden death. N. Eng. J. Med. 346, 1113–1118.
GISSI-Prevenzione Investigators (1999) Dietary supplementation with n−3 polyunsaturated FA and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 354, 447–455.
Charnock, J. S., Abeywardena, M. Y., and McLennan P. L. (1986) Comparative changes in the fatty-acid composition of rat cardiac phospholipids after long-term feeding of sunflower seed oil-or tuna fish oil-supplemented diets. Ann. Nutr. Metab. 30, 393–406.
McLennan P. L., Abeywardena, M. Y., and Charnock J. S. (1988) Dietary fish oil prevents ventricular fibrillation following coronary artery occlusion and reperfusion. Am. Heart. J. 116, 709–717.
Nair S. S., Leitch, J. W., Falconer J., and Garg M. L. (1997) Prevention of cardiac arrhythmia by dietary (n−3) polyunsaturated fatty acids and their mechanism of action. J. Nutr. 127, 383–393.
Pepe S. and McLennan P. L. (1996) Dietary fish oil confers direct antiarrhythmic properties on the myocardium of rats. J. Nutr. 126, 34–42.
Pepe S. and McLennan P. L. (2002) Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation 105, 2303–2308.
Saito M., Ueno M., Kubo K., and Yamaguchi M. (1998) Dose-Response Effect of Dietary Docosahexaenoic Acid on Fatty Acid Profiles of Serum and Tissue Lipids in Rats. J. Agric. Food Chem. 46, 184–193.
Folch J., Lees M., and Sloane Stanley G. H. (1957) A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497–509.
Steck T. L. and Kant J. A. (1974) Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods. Enzymol. 31, 172–180.
Atkinson T. G., Barker H. J., and Meckling-Gill K. A. (1997) Incorporation of long-chain n−3 fatty acids in tissues and enhanced bone marrow cellularity with docosahexaenoic acid feeding in post-weanling Fischer 344 rats. Lipids 32, 293–302.
Gudbjarnason S., Benediktsdottir V. E., and Skuladottir G. (1989) Effects of n−3 polyunsaturated fatty acids on coronary heart disease. Bibl. Nutr. Dieta., 1–12.
Calviello G., Palozza P., Franceschelli P., and Bartoli G. M. (1997) Low-dose eicosapentaenoic or docosahexaenoic acid administration modifies fatty acid composition and does not affect susceptibility to oxidative stress in rat erythrocytes and tissues. Lipids 32, 1075–1083.
Brown, A. P., Dinger, N., and Levine B. S. (2000) Stress produced by gavage administration in the rat. Contemp. Top. Lab. Anim. Sci. 39, 17–21.
Harris W. S., and Von Schacky C. (2004) The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev. Med. 39, 212–220.
Derelanko, M. J. (1987) Determination of erythrocyte life span in F-344, Wistar, and Sprague-Dawley rats using a modification of the [3 H]diisopropylfluorophosphate ([3 H]DFP) method. Fundam. Appl. Toxicol. 9, 271–276.
von Schacky C. and Weber P. C. (1985) Metabolism and effects on platelet function of purified eicosapentaenoic and docosahexaenoic acids in humans. J. Clin. Invest. 76, 2446–2450.
Charnock J. S., Abeywardena M. Y., Poletti V. M., and McLennan P. L. (1992) Differences in fatty acid composition of various tissues of the marmoset monkey (Callithrix jacchus) after different lipid supplemented diets. Comp. Biochem. Physiol. Comp. Physiol. 101, 387–393.
Sexton P. T., Sinclair A. J., O'Dea K., Sanigorski A. J., and Walsh J. (1995) The relationship between linoleic acid level in serum, adipose tissue and myocardium in humans. Asia Pacific J. Clin. Nutr. 4, 314–318.
Harris W. S., Sands S. A., Windsor S. L., Ali H. A., Stevens T. L., Magalski A., Porter C. B., and Borkon A. M. (2004) Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation 110, 1645–1649.
Tavazzi L., Tognoni G., Franzosi M. G., Latini R., Maggioni A. P., Marchioli R., Nicolosi G. L., and Porcu M. (2004) Rationale and design of the GISSI heart failure trial: a large trial to assess the effects of n−3 polyunsaturated fatty acids and rosuvastatin in symptomatic congestive heart failure. Eur. J. Heart Fail. 6, 635–641.
Storlien L. H., Pan D. A., Kriketos A. D., O'Connor J., Caterson I. D., Cooney G. J., Jenkins A. B., and Baur L. A. (1996) Skeletal muscle membrane lipids and insulin resistance. Lipids 31 Suppl., S261-S265.
Di Marino L., Maffettone A., Cipriano P., Sacco M., Di Palma R., Amato B., Quarto G., Riccardi G., and Rivellese A. A. (2000) Is the erythrocyte membrane fatty acid composition a valid index of skeletal muscle membrane fatty acid composition? Metabolism 49, 1164–1166.
Okano G., Matsuzaka H., and Shimojo T. (1980) A comparative study of the lipid composition of white, intermediate, red and heart muscle in rats. Biochim. Biophys. Acta 619, 167–175.
Wu B. J., Hulbert A. J., Storlien L. H., and Else P. L. (2004) Membrane lipids and sodium pumps of cattle and crocodiles: an experimental test of the membrane pacemaker theory of metabolism. Am. J. Physiol. 287, R633-R641.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Owen, A.J., Peter-Przyborowska, B.A., Hoy, A.J. et al. Dietary fish oil dose- and time-response effects on cardiac phospholipid fatty acid composition. Lipids 39, 955–961 (2004). https://doi.org/10.1007/s11745-004-1317-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-004-1317-0