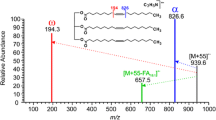
Abstract
Normal-phase HPLC resolution of sn-1,2(2,3)- and x-1,3-DAG generated by partial Grignard degradation from natural TAG was carried out with both (R)-(−) and (S)-(+)-1-(1-naphthyl)ethylurethane derivatives. The diastereomeric sn-1,2- and sn-2,3-DAG derivatives were resolved using two Supelcosil LC-Si (5 μm, 25 cm × 4.6 mm i.d.) columns in series and an isocratic elution with 0.37% isopropanol in hexane at a flow rate of 0.7 mL/min. The DAG were detected by UV absorption at 280 nm and were identified by electrospray ionization MS in the positive ion mode following postcolumn addition of chloroform/methanol/30% ammonium hydroxide (75∶24.5∶0.5, by vol) at 0.6 mL/min. Application of the method to a stereospecific analysis of the molecular species of TAG of rat VLDL showed that the TAG composition of VLDL circulating under basal conditions differs markedly from that of VLDL secreted by the liver during inhibition of serum lipases. The inhibition of serum lipases resulted in a significant proportional decrease in 16∶0 and PUFA and an increase in 18∶0 and oligoenoic FA in the sn-1-position, whereas the FA compositions in the sn-2- and sn-3-positions were much less affected.
Similar content being viewed by others
Abbreviations
- CapEx:
-
capillary exit voltage
- DNPU:
-
dinitrophenylurethane
- HPLC/ESI-MS:
-
HPLC with on-line electrospray ionization MS
- NEU:
-
1-(1-naphthyl)ethylurethane
- 14∶0:
-
myristic acid
- 16∶0:
-
palmitic acid
- 18∶0:
-
stearic acid
- 18∶1:
-
oleic acid
- 18∶2:
-
linoleic acid
- 18∶3:
-
linolenic acid
- 20∶4:
-
arachidonic acid
- 20∶5:
-
eicosapentaenoic acid
- 22∶4:
-
docosatetraenoic acid
- 22∶5:
-
docosapentaenoic acid
- 22∶6:
-
docosahexaenoic acid
References
Michelson, P., Aronsson, E., Odham, G., and Akesson, B. (1985) Diastereomeric Separations of Natural Glycero Derivatives as Their 1-(Naphthyl)ethyl Carbamates by High-Performance Liquid Chromatography, J. Chromatogr. 350, 417–426.
Laakso, P., and Christie, W.W. (1990) Chromatographic Resolution of Chiral Diacylglycerol Derivatives: Potential in the Stereospecific Analysis of Triacyl-sn-glycerols, Lipids 25, 349–353.
Christie, W.W., Nikolova-Damyanova, B., Laakso, P., and Herslof, B. (1991) Stereospecific Analysis of Triacyl-sn-glycerols via Resolution of Diastereomeric Diacylglycerol Derivatives by High-Performance Liquid Chromatography on Silica, J. Am. Oil Chem. Soc. 68, 695–701.
Damiani, P., Santinelli, F., Simonetti, M.S., Castellini, M., and Rosi, M. (1994) Comparison Between Two Procedures for Stereospecific Analysis of Triacylglycerols from Vegetable Oils—I: Olive Oil, J. Am. Oil Chem. Soc. 71, 1157–1162.
Takagi, T., and Itabashi, Y. (1987) Rapid Separations of Diacyland Dialkylglycerol Enantiomers by High-Performance Liquid Chromatography on a Chiral Stationary Phase, Lipids 22, 596–600.
Itabashi, Y., Kuksis, A., Marai, L., and Takagi, T. (1990) HPLC Resolution of Diacylglycerol Moieties of Natural Triacylglycerols on a Chiral Phase Consisting of Bonded (R)-(+)-1-(1-Naphthyl)ethylamine, J. Lipid Res. 31, 1711–1717.
Takagi, T., and Ando, Y. (1991) Stereospecific Analysis of Triacyl-sn-glycerols by Chiral High-Performance Liquid Chromatography, Lipids 26, 542–547.
Taylor, D.C., MacKenzie, S.L., McCurdy, A.R., McVetty, P.B.E., Giblin, E.M., Pass, E.W., Stone, S.J., Scarth, R., Rimmer, S.R., and Pickard, M.D. (1994) Stereospecific Analysis of Seed Triacylglycerols from High Erucic Acid Brassicaceae: Detection of Erucic Acid at the sn-2 Position in Brassica oleracea L. Genotypes, J. Am. Oil Chem. Soc. 71, 163–167.
Folch, J., Lees, M., and Sloane Stanley, G.H. (1957) A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues, J. Biol. Chem. 226, 497–509.
Yang, L.Y., and Kuksis, A. (1991) Apparent Convergence (at 2-monoacylglycerol level) of Phosphatidic Acid and 2-Monoacylglycerol Pathways of Synthesis of Chylomicron Triacylglycerols, J. Lipid Res. 32, 1173–1186.
Yang, L.Y., Kuksis, A., Myher, J.J., and Steiner, G. (1995) Origin of Triacylglycerol Moiety of Plasma Very Low Density Lipoproteins in the Rat: Structural Studies, J. Lipid Res. 36, 125–136.
Brockerhoff, H. (1971) Stereospecific Analysis of Triglycerides, Lipids 6, 942–956.
Itabashi, Y., Myher, J.J., and Kuksis, A. (2000) High-Performance Liquid Chromatographic Resolution of Reverse Isomers of 1,2-Diacyl-rac-glycerols as 3,5-Dinitrophenylurethanes, J. Chromatogr. A 893, 261–279.
Itabashi, Y., Myher, J.J., and Kuksis, A. (1993) Determination of Positional Distribution of Short-Chain Fatty Acids in Bovine Milk Fat on Chiral Columns, J. Am. Oil Chem. Soc. 71, 1177–1181.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ågren, J.J., Kuksis, A. Analysis of diastereomeric DAG naphthylethylurethanes by normal-phase HPLC with on-line electrospray MS. Lipids 37, 613–619 (2002). https://doi.org/10.1007/s11745-002-0940-0
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11745-002-0940-0