Abstract
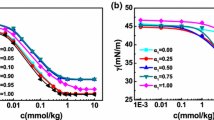
Conductances of hexadecylpyridinium bromide (HPyBr) + tetradecyltrimethylammonium bromide (TTAB) mixtures over the entire mole fraction range of HPyBr (αHPyBr) were measured in pure water as well as in the presence of various aqueous ethylene glycol oligomers containing 10 and 30 wt% of each additive in their respective binary mixtures at 30°C. Each conductivity curve shows two breaks corresponding to two critical micelle concentrations (cmc; C1 and C2 over the whole mole fraction range of HPyBr + TTAB mixtures except in the presence of pure HPyBr and TTAB, where a single break was observed. From the conductivity data, various micellar paramelers in the absence and presence of glycol additives were computed. A variation in the micellar parameters in the presence of additive showed that additive introduction mainly influence the medium properties and therefore the micellar properties. However, no significant micelle-glycol interactions were observed even with an increase in the number of repeating units from ethylene glycol to polyethylene glycol 600. The mixing behavior of HPyBr + TTAB is close to nonideal and is identical in pure water and in the presence of various glycols. This has been attributed to the presence of synergistic interactions between unlike monomers at C1 that are not influenced even by the presence of additives. The appearance of the second cmc is mainly attributed to structural transitions of the mixed micelles at C1 with a further increase in surfactant concentration.
Similar content being viewed by others
References
Tanford, C., The Hydrophobic Effect: Formation of Micelles and Biological Membranes, 2nd edn., Wiley, New York, 1980.
Rosen, M.J., Surfactants and Interfacial Phenomena, John Wiley & Sons, New York, 1989.
Scamehorn, J.F., in Phenomenon in Mixed Surfactant Systems, edited by J.F. Scamehorn, ACS Symp. Ser. 311, 1986, p. 1.
Shinoda, K., and T. Nomura, Miscibility of Fluorocarbon and Hydrocarbon Surfactants in Micelle and Liquid Mixtures. Basic Study of Oil Repellent and Fire Extinguishing Agents, J. Phys. Chem. 84:365 (1980).
Mysels, K., Critical Demicellization Concentration? J. Colloid Interface Sci. 66:331 (1978).
Funasaki, N., and S. Hada, Coexistence of Two Kinds of Mixed Micelles, J. Phys. Chem. 84:736 (1980).
Funasaki, N., and S. Hada, Existence of Critical Demicellization Concentration, J. Colloid Interface Sci. 84:736 (1980).
Bakshi, M.S., Micelle Formation by Anionic and Cationic Surfactants in Aqueous Binary Mixtures, J. Chem. Soc. Faraday Trans. 89:4323 (1993).
Bakshi, M.S., Cetylpyridinium Chloride + Tetradecyltrimethylammonium Bromide Mixed Micelles in Ethylene glycol + Water and Diethylene Glycol + Water Mixtures, —Ibid. 93:4005 (1997).
Bakshi, M.S., G. Kaur, and G. Kaur, Hexadecyltrimethylammonium Bromide + Tetradecyltrimethylammonium Bromide Mixed Micelles in Aqueous Glycol Oligomers, J. Macromol. Sci. A 36:697 (1999).
Bakshi, M.S., and U. Kaur, Tetradecyltrimethylammonium Bromide + Dodecyltrimethylammonium Bromide Mixed Micelles in Aqueous Glycol Oligomers, Bull. Chem. Soc. Jpn. 72:1483 (1999).
Bakshi, M.S., and H. Doe, Cetylpyridinium Chloride + Tetradecyltrimethylammonium Bromide Mixed Micelles in Polyethylene Glycol + Water Mixtures, —Ibid. 72:2041 (1999).
Treiner, C., and A. Makayssi, Structural Micellar Transitions for Dilute Solutions of Long Chain Binary Cationic Surfactant Systems: A Conductance Investigation, Langmuir 8:794 (1992).
Goddard, E.D., Polymer-Surfactant Interaction. Part 1. Uncharged Water-Soluble Polymers and Charged Surfactant, Colloid Surf. 19:255, 301 (1986).
Motomura, K., and M. Aratono, Miscibility in Binary Mixtures of Surfactants, in Mixed Surfactant Systems, edited by K. Ogino and M. Abe, Marcel Dekker, Inc., New York, 1993, p. 15.
Manabe, M., H. Kawamura, Y. Yamashita, and S. Tokunaga, in The Structure, Dynamics and Equilibrium Properties of Colloidal Systems, edited by B.M. Bloor and E. Wyn-Jones, Kluwer Academic Publishers, Dordrecht, 1990, p. 63.
Riddick, J.A., and W.B. Bunger, Organic Solvents, Wiley Interscience, New York, 1970.
Bakshi, M.S., Micelle Formation by Sodium Dodecylsulphate in Water-Additive Systems, Bull. Chem. Soc. Jpn. 69:2723 (1996).
Marangoni, D.G., A.P. Rodenhiser, J.M. Thomas, and J.C.T. Kwak, Solubilization and Aggregation Numbers in Micellar Mixtures of Anionic and Cationic Surfactants with Tetraethylene glycol and Tetraethlene Glycol Momomethylether, Langmuir 9:438 (1993).
Marangoni, D.G., and J.C.T. Kwak, Solubilization of Alcohols and Ethoylated Alcohols in Anionic and Cationic Micelles, —Ibid. 7:2083 (1991).
Evans, H.C., Alkyl Sulphates. Part 1. Critical Micellar Concentration of the Sodium Salts, J. Chem. Soc.:579 (1956).
Rubingh, D.N., in Solution Chemistry of Surfactants, edited by K.L. Mittal, Plenum, New York, 1979, Vol. 1, p. 337.
Clint, J.H., Micellization by Mixed Non-ionic Surface Active Agents, J. Chem. Soc. Faraday Trans. 171:1327 (1975).
Jana, P.K., and P. Moulik, Interactions of Bile Salts with Hexadecyltrimethylammonium Bromide and Sodium Dodecylsulfate, J. Phys. Chem. 95:9525 (1991).
Haque, M.F., A.R. Das, A.K. Rakshit, and S.P. Moulik, Properties of Mixed Micelles of Binary Surfactant Combinations, Langmuir 12:4084 (1996).
Desai, T.R., and S.G. Dixit, Interaction and Viscous Properties of Aqueous Solutions of Mixed Anionic and Cationic Surfactants, J. Colloid Interface Sci. 177:471 (1996).
Nguyen, C.M., J.F. Rathman, and J.F. Scamehorn, Thermodynamics of Mixed Micelle Formation, —Ibid. 112:438 (1986).
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Bakshi, M.S., Kaur, G. Mixed micelles of hexadecylpyridinium bromide + tetradecyltrimethylammonium bromide in aqueous glycol oligomers. J Surfact Deterg 3, 159–166 (2000). https://doi.org/10.1007/s11743-000-0120-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11743-000-0120-5