Abstract
Background
New management, risk stratification and treatment strategies have become available over the last years for patients with acute pulmonary embolism (PE), potentially leading to changes in clinical practice and improvement of patients’ outcome.
Methods
The COntemporary management of Pulmonary Embolism (COPE) is a prospective, non-interventional, multicentre study in patients with acute PE evaluated at internal medicine, cardiology and emergency departments in Italy. The aim of the COPE study is to assess contemporary management strategies in patients with acute, symptomatic, objectively confirmed PE concerning diagnosis, risk stratification, hospitalization and treatment and to assess rates and predictors of in-hospital and 30-day mortality. The composite of death (either overall or PE-related) or clinical deterioration at 30 days from the diagnosis of PE, major bleeding occurring in hospital and up to 30 days from the diagnosis of PE and adherence to guidelines of the European Society of Cardiology (ESC) are secondary study outcomes. Participation in controlled trials on the management of acute PE is the only exclusion criteria. Expecting a 10–15%, 3% and 0.5% incidence of death for patients with high, intermediate or low-risk PE, respectively, it is estimated that 400 patients with high, 2100 patients with intermediate and 2500 with low-risk PE should be included in the study. This will allow to have about 100 deaths in study patients and will empower assessment of independent predictors of death.
Conclusions
COPE will provide contemporary data on in-hospital and 30-day mortality of patients with documented PE as well as information on guidelines adherence and its impact on clinical outcomes.
Trail registration
NCT number: NCT03631810.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute pulmonary embolism (PE) is a common and potentially life-threatening disease [1,2,3,4]. The incidence is estimated to be 0.5–1.5 per thousand person-years [1]. Mortality in patients with acute PE ranges from less than 1% to more than 30% during the hospital stay, depending upon the clinical presentation and comorbidities [5,6,7]. According to currently available evidence, international guidelines recommend to tailor diagnostic workout, patient disposition (intensive care unit vs. medical wards vs. short hospital stay or home treatment) and acute treatment (thrombolysis vs. anticoagulant treatment) to the estimated risk for short-term death [8,9,10,11,12,13,14].
The most recent data on the acute phase management and clinical course in large cohorts of patients with acute PE were published almost 10 years ago [5,6,7]. However, the clinical management and course of PE have been changing over the last years [4]. In fact, new management pathways (home treatment/early discharge), risk stratification tools and treatment strategies (direct oral anticoagulants) have become available with potential influence on patient course and outcome. For these reasons, the assessment of the contemporary clinical management of patients with acute PE would provide valuable information. In addition, assessment in different settings (cardiology, emergency and internal medicine departments) would offer a complete scenario of current management strategies in patients with acute PE.
In this paper, we report the study design of the COntemporary clinical management of patients with acute Pulmonary Embolism (COPE) study in the context of the current literature and recent guidelines.
The aim of the COPE study is to assess contemporary management strategies in patients with acute, symptomatic, objectively confirmed PE concerning diagnosis, risk stratification, hospitalization and treatment and to assess rates and predictors of in-hospital and 30-day mortality. Adherence to guidelines of the European Society of Cardiology (ESC) and its association with in-hospital and 30-day mortality will also be assessed. As this study was planned in 2016 and patient accrual started on 2018, the study protocol has been based on the guidelines of the European Society of Cardiology released in 2014 [10].
Methods
Study design and setting
COPE is a prospective, non-interventional, multicentre study in patients with symptomatic, objectively diagnosed acute PE (either first or recurrent episode) evaluated at Cardiology, Emergency and Internal Medicine Departments in Italy.
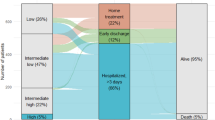
Patients are evaluated at the time of diagnosis, at discharge and at 30 days (± 4) from the index PE (Fig. 1). For patients discharged beyond 30 days from index pulmonary embolism study end -and study outcome assessment- is set at 30 days. Diagnostic workout, risk stratification and treatment strategies are at the discretion and responsibility of the attending physician. Physicians are encouraged to prescribe medications according to their usual standard of care.
COPE is a no-profit study promoted by the University of Perugia and the Fondazione per il Tuo cuore onlus-ANMCO (Associazione Nazionale Medici Cardiologi Ospedalieri), with the collaboration of SIMEU (Società Italiana Medicina Emergenza Urgenza) and FADOI (Federazione delle Associazioni dei Dirigenti Ospedalieri Internisti). The Steering Committee has the full responsibility for the study design, protocol, study oversight, data analysis, and writing and submission of the manuscript for publication. The study is supported by an unrestricted grant from Daiichi Sankyo Europe and Daiichi Sankyo Italia. The study was approved by the Ethic Committees and Institutional Review Boards of the coordinating center and at each participating center.
Participants
Patients 18 years old or older with symptomatic objectively confirmed acute PE are included in the study after release of informed consent in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national).
Criteria for diagnosis of PE are reported in Table 1. For critically ill patients unable to provide the informed consent due to very severe clinical conditions delayed consent is allowed, until more favourable clinical conditions allow them to receive the appropriate information. Patients who die before giving informed consent are also included in the study, upon authorization of local Institutional Review Board. In this case, it is strongly suggested to obtain agreement from relative(s). As this is a non-interventional study, no inclusion/exclusion criteria apply, except for exclusion of patients participating in controlled trials on the management of acute PE.
Approvals and administrative procedures were completed in first trimester 2018 and the first patient in was on April 2018. Patient accrual was completed on first trimester 2021.
Study objectives and outcomes
Study objectives and outcomes are reported in Table 2. The co-primary study outcomes are in-hospital death and death at 30 days from the diagnosis of PE. For all patients, the cause of death is reported as assessed by the attending physician and centrally adjudicated by an independent Critical Event Committee unaware of physician classification.
The secondary study outcomes are (1) death or clinical deterioration at 30 days from the diagnosis of PE; (2) PE-related death or clinical deterioration at 30 days from the diagnosis of pulmonary embolism; (3) adherence to current guidelines on the management of acute PE released by the ESC regarding diagnosis, risk stratification and treatment and (4) major bleeding occurring in-hospital and at 30 days from the diagnosis of PE. The definition of PE-related death is reported in the Table 2.
Study outcomes will be evaluated in the overall study population with descriptive purposes and compared among patients admitted in Cardiology, Emergency or Internal Medicine Departments as well as among patients belonging to different categories of risk according to the ESC guidelines.
Adherence to ESC guidelines 2014 will be assessed concerning: diagnosis, risk stratification, treatment and global management (the composite of diagnosis, risk stratification and treatment). For the assessment of adherence, six process indicators were selected, based on recommendations from ESC guidelines [10]. The indicators are reported in Table 2.
The primary safety outcome is major bleeding according to ISTH criteria, occurring up to 30 days from the diagnosis of index PE (Table 2). Major bleeding events will be further sub-classified as life-threatening or non-life threatening. A life-threatening major bleed is defined as a bleeding event that is either intracranial or is associated with haemodynamic compromise requiring intervention.
Secondary safety outcomes are (a) major bleeding according to ISTH definition plus all bleeding that led to presentation to an acute care facility or hospitalization, occurring up to 30 days from the diagnosis of index PE (b) clinically relevant non-major bleeding defined according to ISTH criteria.
Data sources and measurements
Scheduled assessments for the study are presented in Table 3. All data are collected from information routinely recorded in the patient files/medical records, during clinical visits or during telephone follow-up interviews. All these data are available as part of the routine treatment. All performed examinations depend on the discretion and clinical routine of the physician/site. No diagnostic or monitoring procedures and no examinations, laboratory tests or procedures are applied to the patients as part of this non-interventional study others than those performed as standard of care.
Statistical methods
For all study endpoints, the estimates of overall rates of in-hospital and 30-day events from diagnosis of PE with 95% confidence interval will be calculated.
Incidence rates of the study outcome events will be described and compared in subgroups of patients defined by Department of admission (Cardiology, Internal Medicine, Emergency Medicine) as well as by risk of death category according to the ESC guidelines (high, intermediate and low). Comparisons will be done via Cox proportional hazard regression models presenting hazard ratios and corresponding 95% confidence intervals, having patients admitted at Cardiology or low-risk patients as reference groups, respectively. If differences in baseline characteristics will be detected, these variables will be added to the model as additional covariates. Kaplan–Meier estimates will be calculated for the occurrence of death at 30 days and of death or clinical deterioration at 30 days from diagnosis of PE.
Concerning the definition of adherence to current guidelines, firstly, the number of indicators that each patient met will be counted and divided by the total number for which the patient was eligible, obtaining the proportion of adherence. The adherence score is defined as the ratio of the diagnostic tests/prognostic evaluations/treatment actually prescribed to those that should theoretically have been prescribed. Adherence will be calculated separately for each step (diagnosis/prognostic stratification/treatment) and globally for the overall patient’s management. The theoretical diagnostic/prognostic/treatment/global scores will be calculated for every patient, taking into account guideline-based eligibility criteria, contraindications to tests/drugs or treatments. The adherence score will be calculated for each patient by summing the points attributed as follows: (a) for diagnostic workup: 0 points for use of not recommended diagnostic tests according to pre-test clinical probability; 0.5 points for use of not validated sequence or 1 point for use of recommended sequence of diagnostic tests; (b) for prognostic evaluations: 0 points for no use of tests when indicated; 0.5 points for use of not recommended tests or 1 point for use of recommended tests; (c) for treatment: 0 points for non-prescription of a given treatment (i.e. thrombolysis) in the absence of contraindications, 0.5 points for use of not validated regimen or 1 point for use of validated regimen.
For each step of diagnosis, risk stratification and treatment, the score ranges from 0 (very poor) to 1 (excellent) and we define three levels of adherence: good adherence (score = 1); moderate adherence (score > 0.5 to < 1) and poor adherence (score ≤ 0.5). In this study, the term ‘adherence’ relates solely to physicians following guidelines, not to patient compliance.
We define as adherent to the care pathway a patient with a proportion of met indicators equal or greater than 80%. Sensitivity analyses will be performed at different cut-offs, and considering adherence as an ordinal and a continuous variable. A directed acyclic graph (DAG) will be constructed [14] to represent assumptions regarding the underlying associations between guideline adherence, survival and a set of clinical and socioeconomic variables. The DAG utilizes these assumptions to select the potential confounders, rather than relying simply on the statistical associations observed in the data. The selected confounders are then used in the statistical analysis aiming at minimizing the risk of a biased evaluation of the association between adherence to guidelines and survival.
Sample size
The sample size was calculated to observe at least 100 deaths at 30 days in the study population, to have enough power to describe incidence rates of study outcome events in the overall study population and in patients at high, intermediate and low-risk of death according to ESC guidelines.
Based on the estimated prevalence of patients at high, intermediate and low-risk in a contemporary, prospective cohort of patients with acute PE and expecting an incidence of death at 30 days of 3% and 0.5% for patients with intermediate or low-risk, respectively, it is estimated that 2100 patients with intermediate PE and 2500 with low-risk PE should be included in the study. In the same time frame, it is estimated that around 400 patients with high-risk PE will be observed. The expected mortality in these patients is 10–15%. According to these estimates, we would be able to have about 100 deaths at 30 days in study patients and this will empower assessment of independent predictors of death. Besides already known predictors (hypotension, right ventricle dysfunction—imaging and BNP—and increased troponin), the role of comorbidities and clinical features as well as adherence to current guidelines on PE (risk stratification adequate, appropriateness of acute treatment) as determinants of prognosis will be assessed. The same features will be tested as predictors of death or clinical deterioration.
The inclusion of patients in each risk category (low, intermediate and high risk for death) could be stopped when the predicted number of patients is complete. In that case an alert signal will appear in the e-CRF once the estimated sample is completed.
Discussion
The description of contemporary short-term mortality and management strategies in patients with acute PE is essential to drive future clinical research and inform health systems organization. In this context, the definition of predictors of death and the description of the association between guidelines adherence and clinical course is essential.
Acute PE is a common condition associated with substantial mortality in the short-term after diagnosis. Several international societies released evidence-based guidelines on the management of patients with acute PE [8,9,10,11,12,13,14]. Consensus exists that as in other cardiovascular conditions, clinical management of PE patients in the acute phase should be framed according to disease severity to reduce mortality and optimize resource allocation. In this context, the guidelines from the ESC recommend the use of a well-defined risk-driven patient management in the acute phase, including the use of clinical scores and right ventricle assessment [10, 11]. This approach is not completely endorsed by other guidelines and scientific societies, mainly due to unconvincing evidence that in hemodynamically stable patients with acute PE a risk driven approach is required to adapt clinical management. All guidelines recommend to concentrate special efforts for the management of patients with acute PE presenting in shock or hemodynamic impairment as short-term mortality in these patients can be as high as 30–50%; these patients categorized as at ‘high-risk’ of death according to the ESC Guidelines and as affected by ‘massive PE’ according to the American guidelines should rapidly proceed to a definitive diagnosis and reperfusion by thrombolytic therapy to get reduction in mortality (OR 0.53, 95% CI 0.32–0.88) [15]. The vast majority of patients with acute PE are hemodynamically stable at presentation. In this setting, pre-test clinical probability should drive the diagnostic work-up to avoid useless examinations and exposure to radiation, with acceptable failure rates and risk stratification should drive decision making on hospitalization and acute phase treatment [16,17,18]. However, optimal strategies for risk stratification in this context are controversial [19]. Identification of patients at low risk of death can be made by clinical models or exclusion of right ventricle dysfunction [9,10,11, 19, 20]. Recently, a prognostic role of right ventricle assessment has been confirmed also in PE patients at low-risk according to clinical models [21]. In patients with acute PE and low risk for death, out-patient management (hospitalization < 48 h) has shown safe in clinical studies [22,23,24]; however, the feasibility of out-patient management in clinical practice is debated. Hemodynamically stable patients with evidence of right ventricle dysfunction or injury should be hospitalized as the risk for death in the short term is about twofold that of patients with no signs of right ventricle overload (OR 1.94 95% CI 1.23–3.06 for echocardiography, OR 1.64 95% CI 1.06–2.52 for CT-angiography, OR 5.90 95% CI 2.68–12.95 for increased troponin) [10].
For all patients with acute PE, regardless of risk category for short-term death, anticoagulants are the mainstay for the treatment as these drugs reduce recurrent venous thromboembolism by more than 90% [8,9,10,11,12,13,14]. Direct oral anticoagulants (DOACs) used in fixed doses with no need for monitoring are the new standard for the treatment of venous thromboembolism [25,26,27,28,29,30]. It remains to be addressed whether DOACs can be an appropriate treatment for the overall severity spectrum of patients with acute PE. The real-life adherence to current guidelines on the management of patients with acute PE concerning diagnosis, risk stratification and treatment (including physicians’ preferences on anticoagulation therapies) and whether adherence may influence clinical course is unknown. COPE will provide contemporary evidences on clinical course and guidelines adherence of patients with acute PE in clinical practice.
COPE is a prospective cohort study. The non-interventional design of the study, the potential to include patients in critical conditions or dead and the limited number of exclusion criteria facilitate consecutive enrolment. Randomized clinical trials are essential to assess the role of diagnostic and therapeutic interventions as well as management strategies in a specific clinical setting. The results of these studies are the basis to drive clinical practice guidelines. However, randomized clinical trials generally conducted in selected specialized centers, might not enroll patients who are as heterogenous as those seen in clinical practice. Older and frail patients with contraindications to anticoagulant treatment, patients with severe clinical presentation and multimorbidity continue to be underrepresented in contemporary clinical trials on treatment approaches for patients with acute PE. Registries and other observational data may serve as valuable tools for understanding the level of adherence and the effects of guideline-recommended management in heterogeneous patient populations and settings of care. Central authorities have recently renewed the importance of patient registry studies to collect uniform data on a population defined by a particular disease, condition, or exposure, and that is followed over time [Patient Registry Initiative-Strategy and Mandate of the Cross-Committee Task Force (europa.eu)].
In the COPE study, the participation of Cardiology, Emergency and Internal Medicine Departments allows the inclusion of the whole severity spectrum of acute PE and the evaluation of differences in clinical management among different clinical settings and among academic vs. non-academic hospitals.
For this purpose, study centers were selected to guarantee representativeness for their medical activities as much as possible, and for their interest in the study.
Several large cohort studies and registries have been completed or are currently ongoing in patients with acute venous thromboembolism. All these are prospective, international, cohort studies and none of these are focused on patients with acute PE. The PREFER in VTE disease registry (Prevention of Thromboembolic Events-European Registry in Venous Thromboembolism) was a prospective registry performed in seven European countries to assess the characteristics and the management of patients with venous thromboembolism, the use of health care resources, as well as the costs for 12 months treatment [31]. The study was not focused on patients with acute PE and not on the short-term management and course of the disease. As a consequence, limited data on acute phase management, mortality and decision making are available from this study. Similarly, the Global Anticoagulant Registry in the Field of Venous Thromboembolism Event (GARFIELD-VTE) is an international registry aimed at describing management and outcomes of patients with newly diagnosed deep vein thrombosis and/or PE up to 3 years from venous thromboembolism [32]. The primary objective of this registry is to determine the extent to which the treatment of venous thromboembolism varies in the real-world setting and to assess the impact of such variability on clinical and economic outcomes. The RIETE registry is an ongoing, prospective, dynamic cohort of consecutive patients presenting with symptomatic venous thromboembolism (deep-vein thrombosis, PE, or both) aimed at evaluating the clinical course of patients with acute venous thromboembolism treated with an anticoagulant. RIETE has no definite sample size or critical event committee and is mainly run at Angiology and Internal medicine Departments more than in the Cardiology or Emergency settings. ETNA-VTE is a single arm study on edoxaban treatment in routine clinical practice in patients with venous thromboembolism in Europe [33]. In this context, the focus on the acute phase management of patients with acute PE in a wide spectrum of clinical settings make COPE an original and novel study.
To enhance the scientific validity of our result, we planned to have a Critical Event Committee for central independent assessment of the cause of death based on a pre-defined charter. The Committee is unaware of physician classification and will receive all clinical documents related to the initial PE and to the circumstances of death. The purpose of central study outcome adjudication is to provide standardized, unbiased and blinded evaluation of investigator-reported endpoints, independently from investigator judgement [34]. Critical Event Committees are intended to enhance the scientific validity of a clinical trial through systematic, independent and standardized identification, processing and adjudication of study outcome events [35]. There are multiple lines of evidence indicating that central and independent adjudication of events may affect the results of a randomized trial. The presence of a CEC has been strongly advocated by regulatory authorities and requested in some instances for concern of bias in open-label studies [36, 37].
Conclusion
Acute PE continues to be a leading cause of death and hospitalization all around the world despite continue advances in diagnostic and therapeutic strategies. In this context, contemporary data on in-hospital and 30-day mortality of patients with documented PE as well as information on guidelines adherence and its impact on clinical outcomes are needed to drive clinical research and inform health systems organization.
References
Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B et al (1991) A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 151:933–938
Barco S, Mahmoudpour SH, Valerio L, Klok FA, Münzel T, Middeldorp S et al (2020) Trends in mortality related to pulmonary embolism in the European Region: analysis of vital registration data from the WHO Mortality Database. Lancet Respir Med 8(3):277–287
Bikdeli B, Wang Y, Jimenez D, Parikh SA, Monreal M, Goldhaber SZ et al (2019) Pulmonary embolism hospitalization, readmission, and mortality rates in US older adults, 1999–2015. JAMA 322(6):574–576. https://doi.org/10.1001/jama.2019.8594
Jiménez D, de Miguel-Díez J, Guijarro R, Trujillo-Santos J, Otero R, Barba R et al (2016) Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE Registry. J Am Coll Cardiol 67(2):162–170. https://doi.org/10.1016/j.jacc.2015.10.060
Goldhaber SZ, Visani L, De Rosa M (1999) Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 353:1386–1389. https://doi.org/10.1016/S0140-6736(98)07534-5
Casazza F, Becattini C, Bongarzoni A, Cuccia C, Roncon L, Favretto G et al (2012) Clinical features and short term outcomes of patients with acute pulmonary embolism. The Italian Pulmonary Embolism Registry (IPER). Thromb Res 130(6):847–852. https://doi.org/10.1016/j.thromres.2012.08.292
Pollack CV, Schreiber D, Goldhaber SZ, Slattery D, Fanikos J, O’Neil BJ et al (2011) Clinical characteristics, management and outcomes of patients diagnosed with acute pulmonary embolism in the emergency department. J Am Coll Cardiol 57:700–706. https://doi.org/10.1016/j.jacc.2010.05.071
Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ et al (2011) Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 123:1788–1830. https://doi.org/10.1161/CIR.0b013e318214914f
Giri J, Sista AK, Weinberg I, Kearon C, Kumbhani DJ, Desai ND et al (2019) Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation 140(20):e774–e801. https://doi.org/10.1161/CIRCINTERVENTIONS.120.009353
Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N et al (2014) 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Endorsed by the European Respiratory Society (ERS). Eur Heart J 35(43):3033–3069. https://doi.org/10.1093/eurheartj/ehu283
Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP et al (2020) 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 41(4):543–603. https://doi.org/10.1093/eurheartj/ehz405
Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A et al (2020) American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv 4(19):4693–4738. https://doi.org/10.1182/bloodadvances.2020001830
Stevens SM, Woller SC, Baumann Kreuziger L, Bounameaux H, Doerschug K, Geersing GJ et al (2021) Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report—executive summary. Chest S0012–3692(21):01507–01515. https://doi.org/10.1016/j.chest.2021.07.056
Textor J, Hardt J, Knüppel S (2011) DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology 22(5):745
Chatterjee S, Chakraborty A, Weinberg I, Kadakia M, Wilensky RL, Sardar P, Kumbhani DJ et al (2014) Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 311(23):2414–2421. https://doi.org/10.1001/jama.2014.5990
Carrier M, Righini M, Djurabi RK, Huisman MV, Perrier A, Wells PS et al (2009) VIDAS d-dimer in combination with clinical pre-test probability to rule out pulmonary embolism: a systematic review of management outcome studies. Thromb Haemost 101:886–892
Perrier A, Roy PM, Sanchez O, Le Gal G, Meyer G, Gourdier AL et al (2005) Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 352:1760–1768. https://doi.org/10.1056/NEJMoa042905
van Belle A, Büller HR, Huisman MV, Huisman PM, Kaasjager K, Kamphuisen PW et al (2006) Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, d-dimer testing, and computed tomography. JAMA 295:172–179
Becattini C, Agnelli G, Lankeit M, Masotti L, Pruszczyk P, Casazza F et al (2016) Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J 48:780–786. https://doi.org/10.1183/13993003.00024-2016
Jiménez D, Aujesky D, Moores L, Gómez V, Lobo JL, Uresandi F et al (2010) Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 170:1383–1389. https://doi.org/10.1001/archinternmed.2010.199
Becattini C, Maraziti G, Vinson DR, Ng ACC, den Exter PL, Côté B et al (2021) Right ventricle assessment in patients with pulmonary embolism at low risk for death based on clinical models: an individual patient data meta-analysis. Eur Heart J. https://doi.org/10.1093/eurheartj/ehab329
Aujesky D, Roy PM, Verschuren F, Righini M, Osterwalder J, Egloff M et al (2011) Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet 378(9785):41–48. https://doi.org/10.1016/S0140-6736(11)60824-6
den Exter PL, Zondag W, Klok FA, Brouwer RE, Dolsma J, Eijsvogel M et al (2016) Efficacy and safety of outpatient treatment based on the Hestia Clinical Decision Rule with or without N-terminal pro-brain natriuretic peptide testing in patients with acute pulmonary embolism. A randomized clinical trial. Am J Respir Crit Care Med 194(8):998–1006. https://doi.org/10.1164/rccm.201512-2494OC
Barco S, Schmidtmann I, Ageno W, Bauersachs RM, Becattini C, Bernardi E, HoT-PE Investigators et al (2020) Early discharge and home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban: an international multicentre single-arm clinical trial. Eur Heart J 41(4):509–518. https://doi.org/10.1093/eurheartj/ehz367
Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, EINSTEIN–PE Investigators et al (2012) Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 366:1287–1297. https://doi.org/10.1056/NEJMoa1113572
Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H et al (2009) Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 361:2342–2352. https://doi.org/10.1056/NEJMoa1113697
Schulman S, Kakkar AK, Goldhaber SZ, Schellong S, Eriksson H, Mismetti P et al (2014) Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 129:764–772. https://doi.org/10.1056/NEJMoa0906598
Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M et al (2013) Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 369:799–808. https://doi.org/10.1056/NEJMoa1302507
Hokusai-VTE Investigators, Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S et al (2013) Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 369:1406–1415. https://doi.org/10.1056/NEJMoa1306638
Vedovati MC, Becattini C, Germini F, Agnelli G (2014) Efficacy and safety of direct oral anticoagulants after pulmonary embolism: a meta-analysis. Int J Cardiol 177(2):601–603. https://doi.org/10.1016/j.ijcard.2014.08.136
Cohen AT, Gitt AK, Bauersachs R, Fronk EM, Laeis P, Mismetti P et al (2017) The management of acute venous thromboembolism in clinical practice. Results from the European PREFER in VTE Registry. Thromb Haemost 117(7):1326–1337. https://doi.org/10.1186/s12959-015-0071-z
Haas S, Ageno W, Weitz JI, Goldhaber SZ, Turpie AGG, Goto S, Angchaisuksiri P et al (2019) Anticoagulation therapy patterns for acute treatment of venous thromboembolism in GARFIELD-VTE patients. J Thromb Haemost 17(10):1694–1706. https://doi.org/10.1111/jth.14548
Cohen AT, Ay C, Hainaut P, Décousus H, Hoffmann U, Gaine S et al (2018) Design and rationale of the non-interventional, edoxaban treatment in routiNe clinical prActice in patients with venous ThromboEmbolism in Europe (ETNA-VTE-Europe) study. Thromb J 16:9. https://doi.org/10.1186/s12959-018-0163-7
Petrie MC, McMurray JJV (2020) Do we need clinical events committees to adjudicate end points? Circ Heart Fail 13(7):e007209. https://doi.org/10.1161/CIRCHEARTFAILURE.120.007209
Meah MN, Denvir MA, Mills NL, Norrie J, Newby DE (2020) Clinical endpoint adjudication. Lancet 395(10240):1878–1882. https://doi.org/10.1016/S0140-6736(20)30635-8
Farb A, Zuckerman BD (2017) Clinical event adjudication in cardiovascular device trials: a food and drug administration perspective. Am Heart J 191:62–64. https://doi.org/10.1016/j.ahj.2017.05.010
Lopes RD, Dickerson S, Hafley G, Burns S (2013) Methodology of a reevaluation of cardiovascular outcomes in the RECORD trial: study design and conduct. Am Heart J 166(208–16):e28. https://doi.org/10.1016/j.ahj.2013.05.005
Funding
Open access funding provided by Università degli Studi di Perugia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C.B. reports personal fees from Bayer HealthCare, Bristol Myers Squibb and Daiichi Sankyo all outside the submitted study. G.A. reports personal fees from Pfizer, personal fees from Bayer Healthcare, from Daichi Sankyo, outside the submitted work. A.P.M. reports honoraria for participation in committees of studies sponsored by Bayer, Novartis and Fresenius. M.M.G., A.L., F.D., I.E., F.P., A.F., M.P.R. have nothing to disclose.
Steering committee members
Cecilia Becattini, Michele Gulizia, Giancarlo Agnelli, Aldo P Maggioni, Francesco Dentali, Andrea Fabbri, Iolanda Enea, Fulvio Pomero, Maria Pia Ruggieri, Andrea di Lenarda.
Critical event committee members
Maria Cristina Vedovati, M.D., Chair, Michele Duranti, M.D., Radiologist, Francesco Guercini, M.D., Paolo Groff, M.D., Melina Verso, M.D.
Statement of human and animal rights
All study procedures were in accordance with National and International regulations on Ethical standards for clinical research.
Informed consent
All patients provided informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Becattini, C., Agnelli, G., Maggioni, A.P. et al. Contemporary clinical management of acute pulmonary embolism: the COPE study. Intern Emerg Med 17, 715–723 (2022). https://doi.org/10.1007/s11739-021-02855-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-021-02855-0