Abstract
Variation in root system architecture (RSA) and morphology are important for improving phosphorus use efficiency (PUE) in wheat. This work aims to analyze the root system architecture and morphology of wheat genotypes to explain their variation in PUE. Two genotypes differing in PUE, RAC875 (P efficient) and Wyalkatchem (P inefficient) were grown in a sandy soil amended with adequate basal nutrients and two P rates (10 and 30 mg P kg−1). RSA traits were measured by freely available software, GiA roots and DIRT, and root hair features were measured using a microscope with an attached camera and LAS v3.6 software. Under low P supply, RAC875 produced greater shoot dry matter (DM) at 24, 27 and 48 days after sowing (DAS), and at maturity, RAC875 also had a higher grain yield at maturity. Enhanced P efficiency (biomass and seed yield at inadequate P supply relative to adequate P supply) was observed more so in RAC875 at all harvest times. P supply affected most RSA traits, with low P leading to reductions in convex hull area (CHA), root surface area, root volume, total root length and root tip number. RAC875 produced significantly greater CHA than Wyalkatchem at low P supply while Wyalkatchem had significantly larger CHA than RAC875 when P was non-limiting. RAC875 also had greater root hair density (RHD) than Wyalkatchem at low P level, but no difference was observed at adequate P. When grown under low P supply, a greater CHA and RHD in RAC875 were likely to contribute to improved P uptake, resulting in its higher shoot biomass and grain yield.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As roots are the conduit for mineral and water acquisition from soils, they are logically a target for manipulation to improve crop productivity on soils with poor nutrition (Meister et al. 2014). Root system architecture (RSA) and morphology are factors used to define properties of root systems; therefore, it is vital to identify their favorable traits for the improvement of crop yield. Root morphology refers to the features of a single root axis as an organ, including root hairs, root diameter and cortical senescence. RSA relates to the whole root system or a large subset of the root system and may be described as topological or geometric measures of the root shape (Lynch 1995; Bucksch et al. 2014). Wheat has the monocot root system that consists of several seminal roots and adventitious roots (Sinha et al. 2018). Traits often used for wheat roots are total root length, root surface area, root volume, root angle, number of roots and root diameter (Ahmadi et al. 2018; York et al. 2018). Although a number of platforms have been developed for root phenotyping, genetic studies on root traits are hindered due to their complexity, underground location and interactions with the environment (Kuijken et al. 2015; Paez-Garcia et al. 2015; Topp et al. 2016). Therefore, suitable methods need to be developed to explore root traits.
Current efforts to study the structure of crop root systems have resulted in a number of root phenotyping platforms that are able to elucidate RSA under various conditions, including laboratory, greenhouse and field conditions (Paez-Garcia et al. 2015). RSA phenotyping requires a growth system, root imaging system and software tools. A variety of software tools has been developed for RSA characterization including manual, semi-automatic and automatic programs (Lobet et al. 2015). Root images can be obtained with or without the destruction of root systems, depending on the cultivation method and imaging techniques. Each method has its own advantages and disadvantages. Using a gel-based growth platform, roots are visible and can be easily imaged in situ (Iyer-Pascuzzi et al. 2010), but root behavior in gel may not be comparable to root behavior in soils. In contrast, intact root systems cannot be imaged in soil growth systems as roots need to be extracted before being imaged (da Silva et al. 2016); therefore, some root traits such as root angle and CHA are not able to be accurately measured. Although advanced techniques such as X-ray (Mooney et al. 2012) or magnetic resonance imaging (MRI) (van Dusschoten et al. 2016) can be applied for non-destructive imaging in soils, these are expensive approaches. Glass bead-based cultivation in specialized rhizoboxes is a good approach to retain two-dimensional (2D) RSA in rice (Courtois et al. 2013), but this method does not use soil-based cultivation. Thus, the development of specialized soil-based growth rhizoboxes to obtain root systems without destruction is ideally needed for RSA analysis.
Phosphorus (P) deficiency occurs in the majority of terrestrial ecosystems and reduces crop productivity (Shenoy and Kalagudi 2005; Lynch 2011). This sub-optimal supply can be overcome through the application of P fertilizers (Shepherd et al. 2016); however, this is only a part of the solution because P fertilizers are non-renewable, potentially harmful to the environment when in oversupply and costly (Jones et al. 1989; Liu 2015; Roberts and Johnston 2015). Therefore, the development of crops that harbor an enhanced ability to acquire P as well as to utilize P more efficiently is an important strategy to improve agricultural productivity (Vance et al. 2003; Lambers et al. 2006; Lynch 2007; Wang et al. 2010).
In response to P deficiency, plants may display a variety of adaptation mechanisms, one of which is modification of RSA/morphology (Zhu et al. 2006; Niu et al. 2013). Indeed, plants can adjust their root systems to P stress via stimulation of root and lateral root growth (Gaume et al. 2001; Zhu et al. 2005a), enhancement of root hair development (Foehse and Jungk 1983; Bates and Lynch 1996) and formation of cluster root (Wasaki et al. 2003; Abdolzadeh et al. 2010). The increase in root hair length and root hair density under low P was observed in wheat (Wang et al. 2016b). Genotypic variation in RSA and morphology under P starvation has been observed in food crops. For example, studies in wheat have shown that root hair length and density correlate with P acquisition (Gahoonia et al. 1997; James et al. 2016). Wheat genotypes with a shallow root system exhibit greater P uptake efficiency (da Silva et al. 2016). Also, maize genotypes with shallow root systems show greater growth and P accumulation than deep-rooted genotypes under low P conditions (Zhu et al. 2005b). Bean genotypes with longer root hairs and shallow roots produced significantly greater biomass than short-haired, deep-rooted genotypes (Miguel et al. 2015). Root traits including root surface area, root volume and root length were moderately heritable in maize under low P supply (Zhang et al. 2014). Once heritable traits associated with PUE are identified, they can be used to generate more P-efficient crops through plant breading or genetic modification.
Genotypic variations that are linked with yield improvements and mechanisms for coping with limited P supply are an important area of study. Modification of root architecture is one of the mechanisms for plants to cope with P stress; however, previous wheat RSA studies have had technical limitations (as highlighted above). The aim of this study was to develop a simple, non-destructive method for studying the RSA of wheat grown in soil to characterize RSA traits of two genotypes differing in PUE.
Materials and methods
Wheat varieties
Two wheat genotypes, RAC875 and Wyalkatchem, were used for the experiments. RAC875 is a breeding line and Wyalkatchem is a cultivar grown in Australia. From field experiments (McDonald et al. 2015) and our own preliminary results, RAC875 was P efficient and Wyalkatchem was P inefficient.
Experiment 1. Genotypic evaluation for P use efficiency
Experimental design and plant growth conditions
Plants were grown in round pots (dimensions: 18.5 cm deep × 17.5 cm top diameter × 16.0 cm base diameter) filled with 4.2 kg of soil at two P rates: 10 and 30 mg P kg−1. Basal nutrients (expressed in mg kg−1) consisting of Ca(NO3)2·4H2O (918), K2SO4 (113.6), MgSO4·7H2O (140), FeSO4·7H2O (1.4), Na2MoO4·2H2O (0.61), CuSO4·5H2O (2.25), MnSO4·4H2O (3.68), ZnSO4·7H2O (6.6), and H3BO3 (0.28) were added and mixed thoroughly into the soil. P (in the form of KH2PO4) was then added at two different rates; 10 and 30 mg P kg−1 (low and adequate P, respectively) and mixed thoroughly into the soil.
Wheat grains were sterilized in 2% hypochlorite for 10 min and then rinsed with Milli-Q water. The sterilized grains were then placed in Petri dishes lined with moistened filter papers and kept in a dark place at room temperature for 3 days. Five germinated grains were sown into each pot and the experiment was conducted with four replicates in a controlled environment growth room with the following conditions: 20 °C/10 °C, 13-h/11-h day/night cycle, and light intensity of 700 µmol m−2 s−1 at the leaf level. Light source was a combination of fluorescent and incandescent. Pots were arranged in a completely randomised design and were rotated every 3–7 days to minimize the effect of light gradient within the growth room. The plants were watered to 8–10% of the soil weight every 2–3 days.
Harvest and measurements
Two plants per pot were harvested at 27 days after sowing (DAS), one plant was harvested at 48 DAS and the remaining two plants were grown to maturity. At maturity, wheat heads were separated from shoots and stems were detached from roots at the crown. Shoots and stems were rinsed with Milli-Q water and dried for 48 h at 85 °C for dry matter measurements. Heads were dried at 37 °C for 5 days and grains collected using a Haldrup thresher for measurements of grain yield. The dry mass of roots was also measured after drying at 85 °C for 48 h.
Experiment 2. RSA characterization
The rhizoboxes used in this study were based on the design of those in the Kono et al. (1987) and Courtois et al. (2013) study with modifications. The rhizoboxes have three main parts: a plastic box (L × W × D: 29.5 × 20 × 4 cm) with holes for watering, a foam base with a grid of toothpicks to maintain the root system architecture and a transparent plastic sheet to sit under the root system (Fig. 1).
Plants were grown in rhizoboxes filled with 1.2 kg of double-washed sandy soil with added basal nutrients as described in the previous experiment with two P rates: 10 and 30 mg P kg−1. Wheat grains were also sterilized and germinated as described in the previous experiment, and one germinated seed was sown into each rhizobox. The experiment was carried out in triplicates in a growth room with the conditions as described in the previous experiment, except light intensity was 500 µmol m−2 s−1. The rhizoboxes were kept at a 60° angle for the duration of the experiment and plants were watered every 2–3 days to 8% of the soil weight.
Plants were harvested at 24 DAS and shoots were detached from roots at the crown level. Soil was gently washed away by slow agitation in a tank of water; the toothpicks within the rhizobox minimize the root movement during washing. The transparent sheet with the root system was removed from the rhizobox and the root system was gently blotted with absorbent paper and scanned (8-bit gray scale, 400 dpi) on an Epson Perfection V700 Photo scanner for RSA analysis. Global RSA traits were measured using GiA Roots (Galkovskyi et al. 2012) and other RSA traits were measured using the DIRT software program (Bucksch et al. 2014). GiA roots were used to measure convex hull area (CHA, the smallest area that encloses the whole root system), root surface area, total root length and root volume. DIRT was used to measure root tip number, medium root width, root density, root angle, spatial root distribution (displacement of the center of mass between the bounding box of the RTP skeleton and the RTP skeleton excluding the central path; the RTP skeleton is a loop-free sampling of the medial axis derived from the root shape visible in the image) and accumulated width over the depth (D).
Shoot dry matter (Shoot DM) was measured after 3 days of freeze-drying and root dry matter (root DM) was measured after 48 h at 85°C to analyze the correlation between root traits and shoot DM.
Experiment 3. Root hair characterization
Wheat grains were grown in transparent plastic rootboxes filled with 600 g of sandy soil, adding basal nutrients as described in the previous experiment, and at two P rates of 10 and 30 mg P kg−1 soil. The rootbox size was L × W × D: 24 × 24 × 2.5 cm. The experiment was carried out in four replicates in a growth room as described in the previous experiment, except light intensity was 650 µmol m−2 s−1 at the leaf level. Rootboxes were diagonally placed at a 45° angle, to ensure roots attached to the surface of the rootbox. Soil was moistened to 10% of the soil weight. Seminal root diameter, root hair length (RHL) and root hair density (RHD) on the longest seminal roots were measured at 5 DAS using a microscope (LEICA MZ16, × 12.5 and × 20 magnifications) with an attached camera (LEICA DFC280) and LAS v3.6 software. Images of roots and root hairs were observed through transparent plastic rootboxes and captured within 2–4 cm from the root tip. Eight measurements from each replicate were taken for seminal root diameter and RHL. The root hair density (RHD) was estimated by the equation described by Vandamme et al. (2013): RHD = (πr 2s )−1, in which rs is the half-mean distance between the root hairs, measured by counting the number of root hairs per 0.5 mm of root length. Four measurements were taken from each replicate.
Analysis and calculations
The P concentration in shoot, grain and straw was determined by inductively coupled plasma mass spectrometry (ICP-MS) (Agilent Technologies, Model 7500cx) at Flinders University using the closed-tube digestion method developed by Wheal et al. (2011). PUE of wheat genotypes was evaluated by five criteria as described below (Osborne and Rengel 2002).
-
(1)
Shoot biomass and grain yield at low P.
-
(2)
\({\text{PUE}}_{\text{SM}} = \frac{\text{Shoot DM at low P}}{\text{Shoot DM at adequate P}} * 1 0 0.\)
-
(3)
\({\text{PUE}}_{\text{GY}} = \frac{\text{Grain yield at low P}}{\text{Grain yield at adequate P}} * 1 0 0.\)
-
(4)
P acquisition efficiency (PAE): \({\text{PAE}} = \frac{\text{Shoot P uptake}}{\text{Amount of P supplied}} * 1 0 0.\)
-
(5)
P utilization efficiency (PUtE): \({\text{PUtE}} = \frac{\text{Shoot DM}}{\text{Shoot P uptake}}.\)
Statistical analysis
Statistical analyses were conducted in IBM SPSS v23. The normality of data was tested using Kolmogorov–Smirnov and Shapiro–Wilk tests (P < 0.05). P uptake and root to shoot ratio were not normally distributed and were transformed using log10. Plant indices and root architectural traits were analyzed by two-way ANOVA (Genotype × P supply). Root hair features were analyzed by three-way ANOVA (Genotype × Growth stage × P supply). Mean comparisons between genotypes at each P treatment were performed by independent t-test (P < 0.05). The particular sets of variables were subjected to Pearson’s correlation analysis (Field 2013).
Results
Genotypic evaluation for P use efficiency
Shoot DM at 27 DAS slightly increased with the addition of P, but significant (P < 0.01) responses to P occurred at 48 DAS and at maturity (Table 1). When compared to Wyalkatchem, RAC875 produced significantly (P < 0.05) greater shoot DM at 27 DAS, 48 DAS and at maturity. No significant G × P interaction was observed. Under low P availability, RAC875 had significantly (P < 0.05) greater shoot DM at all three growth stages and higher grain yield than Wyalkatchem. No significant genotypic variation for these traits was found under adequate P supply (Table 1). PUESM of RAC875 was 11, 3 and 15% higher than those in Wyalkatchem at 27 DAS, 48 DAS, and at maturity, respectively. RAC875 also showed 17% greater PUEGY than Wyalkatchem (Table 2).
At maturity, RAC875 had a significantly (P < 0.01) smaller root to shoot ratio and a smaller (P < 0.001) root DM than Wyalkatchem (Table 3). At this growth stage, under low P supply, P uptake of RAC875 was significantly (P < 0.01) higher than that of Wyalkatchem, while no difference in this variable between the two genotypes was observed at adequate P. RAC875 exhibited higher root efficiency than Wyalkatchem under low P supply with root efficiency of RAC875 almost double that of Wyalkatchem (Table 3).
RSA characterization and responses to P of two wheat genotypes grown in rhizoboxes
RSA traits
Results indicate that P supply did not significantly affect root angle, average root diameter, spatial root distribution (X) (RDISTRx) and average density (Table 4). However, low P resulted in significant reductions in CHA, total root length, root surface area, root volume, root tip number, medium root with, and the absolute value of spatial root distribution (Y) (RDISTRy) (Table 4).
On low-P soils, RAC875 showed a moderate 11.2% decrease in CHA, a 14.5% decrease in total root length, a 16% decrease in root surface area, a 17.2% decrease in root volume, a 22.3% decrease in root tip number and a 14.8% decrease in medium root width, and showed no decline in the absolute value of spatial root distribution (Y). Meanwhile, Wyalkatchem had a marked 43.5% reduction in CHA, 47% reduction in total root length, 47.2% reduction in root surface area, 48.5% reduction in root volume, 41.5% reduction in root tip number and 44.8% reduction in medium root width, and also showed no significant decrease in the absolute value of spatial root distribution (Y) (Table 4, Fig. 2). There were no significant variations in RSA traits between the two genotypes; however, the mean ratios between low P and adequate P for total root length, root surface area, root volume, root tip number and medium root width were 32.5, 31.2, 31.3, 19 and 30%, respectively, greater in RAC875 than in Wyalkatchem (Table S 1).
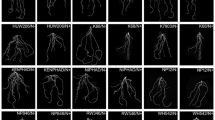
Convex hull area of two wheat genotypes RAC875 and Wyalkatchem grown in rhizoboxes at 24 DAS. Data were the means ± standard errors (n = 3). *, ** significant within the same P supply at P < 0.05, P < 0.01, respectively. Mean comparisons between genotypes at each P treatment were performed by independent t-test. Root system architecture of RAC875 and Wyalkatchem grown in the rhizoboxes at two P rates (P10 and P30: 10 and 30 mg P kgle 5); representative photos show RAC875 (left) and Wyalkatchem (right) at 24 DAS grown under low P levels (10 mg P kg−1)
A significant G × P interaction for CHA was observed. At low P supply, CHA in RAC875 was 16.1% larger than that in Wyalkatchem (P < 0.05), but 19.4% smaller at adequate P supply (P < 0.01) (Fig. 2)
No genotypic variation in specific accumulated width (D) over depth was observed under low P supply, except at D90 where RAC875 showed a significantly (P < 0.05) greater accumulated width over depth when compared to Wyalkatchem. Under adequate P supply, D values in Wyalkatchem were higher than those in RAC875 (Fig. 3).
Accumulated root width over root depth (%) for two wheat genotypes, RAC875 and Wyalkatchem at a low P supply (10 mg P kg−1) and b adequate P supply (30 mg P kg−1). Data were the means ± standard errors (n = 3). *Significantly different between genotypes at specific depth (P < 0.05). Mean comparisons between genotypes at each P treatment were performed by independent t-test
Responses to P and PUE of two wheat genotypes
Shoot DM was significantly affected by P supply at 24 DAS. Shoot DM at adequate P was about 74% greater than that at low P supply (10 mg P kg−1) (P < 0.01) (Table S2). Under low P condition, shoot DM in RAC875 was 56% higher than Wyalkatchem (P = 0.07), while there was no difference between these two wheat genotypes at adequate P supply (Fig. 4). Consequently, RAC875 showed 55% higher PUESM than Wyalkatchem.
A significant G × P interaction in shoot P concentration was observed (P < 0.05) (Table S2), indicating these wheat genotypes behaved differently at different P levels. Indeed, shoot P concentration in Wyalkatchem was slightly higher than that in RAC875 at low P supply, while at adequate P supply, Wyalkatchem showed significantly (P < 0.05) higher shoot P concentration (Table S2).
PUtE significantly (P < 0.001) declined with an increase in P supply and RAC875 showed significantly greater PUtE than Wyalkatchem (Table S2). No significant differences in P uptake, PAE and root efficiency were observed between the two wheat genotypes, but RAC875 was generally higher in these parameters under low P supply (Table S2).
Shoot P uptake per unit of RSA traits was calculated to evaluate how the RSA traits affect shoot P uptake (Table 5). Under low P supply, a significant reduction occurred in shoot P uptake per unit of root trait (including CHA, root surface area, root volume, total root length and root tip number); however, significant differences in these parameters were not found between genotypes. On low-P soils, shoot P uptake per unit of average root density in RAC875 was significantly (P < 0.05) higher than in Wyalkatchem but no variation in this parameter was observed between the two wheat genotypes in adequate-P soils (Table 5).
Root hair length and density, and seminal root diameter
For the estimation of root hair features, a preliminary trial showed that root hairs of wheat were sparse and delicate, and they were mostly lost or were aggregated when harvested from the pot experiment. Thus, growing wheat in transparent rootboxes for root hair measurements is a viable approach. The results illustrated that root hairs were well observed and captured through the transparent rootboxes under a microscope with a camera attached (Fig. 5).
Variations in seminal root diameter (a), RHL (b) and RHD (c) of two wheat genotypes, RAC875 and Wyalkatchem, under different P supply. Results were means ± standard errors (n= 4). *Significant differences P < 0.05 between genotypes grown under the same P supply at each growth stage. Mean comparisons between genotypes at each P treatment were performed by independent t-test. The root picture shows examples of two representative root systems of RAC875 (left) and Wyalkatchem (right) at low P supply (10 mg P kg−1)
Results in Table 6 and Fig. 5a show that RAC875 had significantly (P < 0.001) greater (P < 0.001) seminal root diameter than Wyalkatchem, while P supply did not affect the seminal root diameter. Both genotype and P supply did not have a significant impact on RHL (Table 6, Fig. 5b). At low P supply, RAC875 showed significantly (P < 0.05) higher RHD than Wyalkatchem, while no difference in RHD was observed at adequate P supply (Fig. 5c).
Discussion
Responses to P and PUE of two wheat genotypes
The consistency in response to P was observed between plants grown in the rhizoboxes and in pots as well as in the field, therefore, the RSA traits were measured from the roots grown in the rhizoboxes could be used in practice. Field trial experiments showed that RAC875 produced high grain yield under low P (McDonald et al. 2015), and RAC875 exhibited greater PUE than Wyalkatchem (PUE was calculated from supplemental data provided by McDonald). In this study, under low P, RAC875 also showed greater shoot DM at different growth stages (27 DAS, 48 DAS and at maturity) and higher grain yield than Wyalkatchem in the pot experiment under growth room conditions (Table 1). PUE of RAC875 was also higher than that of Wyalkatchem at all three growth stages (Table 2). Similar results were also observed in the rhizobox experiment (Fig. 4). The consistency of the results indicates that the rhizoboxes are a suitable soil-based method to elucidate the root RSA traits of wheat.
Lower P requirement for its normal growth would allow RAC875 to have a higher PUtE. At maturity and under low P supply, P uptake in RAC875 was 30.7% greater than that in Wyalkatchem (Table 3) and this value was also higher in RAC875 than in Wyalkatchem at 24 DAS (Table S2). Wyalkatchem had a significantly higher shoot P concentration when compared to RAC875, although it produced less biomass at 24 DAS (Table S2). This would indicate that Wyalkatchem requires more P for its normal growth in comparison with RAC875. Furthermore, RAC875 utilized P more efficiently than Wyalkatchem since PUtE in RAC875 was significantly higher than that in Wyalkatchem (Table S2).
Plants with a smaller root system would provide more energy for shoot growth. In contrast to a previous study in coffee plants where high root to shoot ratio was positively associated with efficiency in P uptake (Neto et al. 2016), in this study, the root to shoot ratio in RAC875 was significantly smaller than that in Wyalkatchem. RAC875 also has a smaller root biomass than Wyalkatchem at maturity (Table 3). In agreement with these findings, da Silva et al. (2016) also reported that P-efficient wheat genotypes possessed smaller root systems than P-inefficient genotypes. It appears that plants with smaller root systems may require less energy for root growth; therefore, they can sustain biomass under limited P nutrition. However, a question arises as to how small a root system can be to maintain shoot biomass under P deficiency, and a critical level study would be beneficial to determine this. A study on a large set of germplasm can provide an answer to this question.
RSA responds to P supply
Growth methods and conditions as well as harvest stage could affect RSA trait measurements. In our study, at 24 DAS, a sub-optimal level of P supply led to a significant reduction in CHA, total root length, root surface area, root volume, root tip number, medium root width, and the absolute value of spatial root distribution (Y) (Table 4). Similarly, low P resulted in a decrease in total root length, root surface area and root tip number in barley at 28 DAS, under hydroponics (Wang et al. 2015). However, enhanced root length under low P was observed in wheat grown in a hydroponic experiment (Horst et al. 1993) and in barley grown in a field experiment (Steingrobe et al. 2001). The variations in results could be due to differences in harvest stages and in methodologies [i.e. this study used a soil-based cultivation in rhizoboxes and the plants were harvested at 24 DAS, while Horst et al. (1993) examined root traits at 14 DAS in a hydroponic experiment; Steingrobe et al. harvested only the 0–30 layers in the field but not the whole root system]. It appears that at a very early stage of growth, plants under low P stimulate greater root growth to obtain more P and their root length is higher than under adequate P. However, when plants grow longer, plants under adequate P grow larger and result in a larger root system, as our results indicate.
Larger CHA could help to explain the greater persistence under abiotic stress. Although previous studies show that topsoil foraging is advantageous for phosphorus acquisition in common bean and maize under low P supply (Lynch and Brown 2001; Zhu et al. 2005b), in this study root top angle did not vary between the two wheat genotypes (Table 4). Thus, RAC875 could have different mechanisms of PUE. Indeed, under P deficiency, RAC875 had significantly larger CHA than Wyalkatchem, while the result was reversed under adequate P supply (Fig. 4). Therefore, the mechanism of PUE seems to be interesting in this case. Under limited P supply, the root system of RAC875 expanded and occupied a larger area than that of Wyalkatchem. RAC875 produced higher shoot DM than Wyalkatchem under low P supply. This may help explain a mechanism for better growth and P uptake of RAC875 under low P and thus CHA appears to be a potential indicator for screening P-efficient wheat under low P. Large CHA was a characteristic of drought tolerance in wheat (Belachew et al. 2018). A study by Kenobi et al. (2017) also indicated that CHA can be used as a discriminant to identify high-nitrogen uptake efficiency in wheat genotypes.
Root hairs respond to P and relations between root hair features and PUE
Root hairs are important traits for improvement of PUE since their characteristics affect P acquisition (Gahoonia et al. 1997; Gahoonia and Nielsen 2003; Haling et al. 2013). Although previous studies have demonstrated that root hairs became longer under low P, such as in Arabidopsis thaliana (Bates and Lynch 2000), maize (Zhu et al. 2010) and rice (Vejchasarn et al. 2016), this study showed that P supply did not affect RHL (Table 6). This difference might simply be attributed to genetic differences between these plants. A recent study also indicated that an increase in P supply resulted in improved RHL in wheat (Yuan et al. 2016). Obviously, variation in observed results between research groups would indicate that a large number of genotypes should be used for the evaluation of plant adaptation to low P, by promoting RHL.
The role of RHL in PUE is still not obvious. In the study presented here, RHL did not show any contribution to higher PUE in RAC875 because RAC875 and Wyalkatchem were not different in RHL (Table 6, Fig. 5b). Brown et al. (2012) also pointed out that RHL is not important for grain yield but for shoot P accumulation. However, Gahoonia and Nielsen (2004) have shown that barley genotypes with long root hairs improved grain yield in comparison with short root hair genotypes. Recently, quantitative trait loci (QTLs) for RHL have been identified in wheat and they co-located with loci for yield components (Horn et al. 2016). Thus, a population study should be implemented to identify if RHL is important for grain yield under low P.
Dissimilar to RHL, RHD is not only responding to low P supply but also related to PUE. In this study, RHD increased (P = 0.063) when P supply was low (Table 6, Fig. 5c) and this result agrees with a number of studies (Bates and Lynch 2000; Ma et al. 2001; Hill et al. 2010; Hu et al. 2010). A previous study was able to show that higher RHD enhanced P uptake under low P in wheat (Wang et al. 2016a) and this could be important for later plant growth. Similarly, in this study, RAC875 produced significantly greater RHD than Wyalkatchem under low P but not under adequate P, indicating that more dense root hairs could contribute to greater shoot DM and yield in RAC875 in low P conditions.
What root features could be associated with PUE in RAC875?
Root efficiency can be considered to be involved in greater PUE. da Silva et al. (2016) found that root volume was negatively correlated with PUE in wheat. It appears that the efficiency of a root system to acquire P is more important than the actual root size. Root efficiency is calculated as mg shoot P uptake per unit of g root dry matter or root surface area (Mori et al. 2016). According to Jones et al. (1989), root efficiency is an indication as to the fineness and structure of a root system and its soil explorative capacity, and it may be used in breeding programs for improved P efficiency in wheat. Studies in rice have shown that genotypes with high root efficiency (in this case, calculated as shoot P uptake per unit of root surface area) had greater P uptake efficiency (Wissuwa 2005; Mori et al. 2016). In this study, RAC875 was shown to have greater root efficiency and to produce higher yield under low P than Wyalkatchem. Thus, root efficiency would be important for screening P-efficient genotypes.
The increased root efficiency of RAC875 could be related to greater CHA and more dense root hairs. Indeed, under low P supply, CHA in RAC875 was larger than that in Wyalkatchem (Fig. 2). Also, under low P, RAC875 had greater root hair density than Wyalkatchem (Fig. 5). Keyes et al. (2013) reported that roots and root hairs equally contribute to P uptake, in which root hairs are more important for localized P acquisition. Therefore, it appears that the enlargement of a root system and the development of more root hairs under low P ensure that the P-efficient genotype with a small root system (in terms of root dry matter) can support higher yield production.
Conclusions
In summary, the specially designed rhizoboxes can be used to grow wheat in soils and obtain root systems without destruction for RSA analysis. Larger CHA and more dense root hairs appear to lead to enhanced shoot DM and grain yield in RAC875 under low P conditions. These root characteristics would ensure that a small root system as in RAC875 can support relatively greater biomass and yield production. Thus, it seems that small but efficient root systems would be a beneficial indicator for screening P-efficient crops. Root surface area, total root length and root volume would contribute to high productivity of RAC875 on low-P soils since these parameters were positively correlated with shoot DM and shoot P uptake. A larger screening of genotypes is also recommended to further validate this screening technique and its application in marker development could also be realized with a dedicated marker discovery study in a suitable mapping population.
Author contribution statement
VLN and JS designed the research. VLN implemented the experiments and performed the data analyses. VLN wrote the manuscript. JS made the revision of the manuscript. All the authors approved the final revision to be published.
Abbreviations
- CHA:
-
Convex hull area
- DAS:
-
Day after sowing
- DM:
-
Dry matter
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- P:
-
Phosphorus
- PAE:
-
Phosphorus acquisition efficiency
- PUE:
-
Phosphorus use efficiency
- PUEGY :
-
Phosphorus use efficiency calculated as the ratio of grain yield under low P to grain yield under adequate P
- PUESM :
-
Phosphorus use efficiency calculated as the ratio of shoot matter under low P to shoot matter under adequate P
- PUtE:
-
Phosphorus utilization efficiency
- RSA:
-
Root system architecture
- RHD:
-
Root hair density
References
Abdolzadeh A, Wang X, Veneklaas EJ, Lambers H (2010) Effects of phosphorus supply on growth, phosphate concentration and cluster-root formation in three Lupinus species. Ann Bot 105:365–374
Ahmadi J, Pour-Aboughadareh A, Fabriki-Ourang S, Mehrabi A-A, Siddique KHM (2018) Screening wheat germplasm for seedling root architectural traits under contrasting water regimes: potential sources of variability for drought adaptation. Arch Agron Soil Sci 64:1351–1365
Bates TR, Lynch JP (1996) Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ 19:529–538
Bates TR, Lynch JP (2000) The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. Am J Bot 87:964–970
Belachew KY, Nagel KA, Fiorani F, Stoddard FL (2018) Diversity in root growth responses to moisture deficit in young faba bean (Vicia faba L.) plants. PeerJ 6:e4401
Brown LK, George TS, Thompson JA, Wright G, Lyon J, Dupuy L, Hubbard SF, White PJ (2012) What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Ann Bot 110:319–328
Bucksch A, Burridge J, York LM, Das A, Nord E, Weitz JS, Lynch JP (2014) Image-based high-throughput field phenotyping of crop roots. Plant Physiol 166:470–486
Courtois B, Audebert A, Dardou A, Roques S, Ghneim-Herrera T, Droc G, Frouin J, Rouan L, Gozé E, Kilian A, Ahmadi N, Dingkuhn M (2013) Genome-wide association mapping of root traits in a Japonica Rice panel. PLoS One 8:e78037
Da Silva A, Bruno IP, Franzini VI, Marcante NC, Benitiz L, Muraoka T (2016) Phosphorus uptake efficiency, root morphology and architecture in Brazilian wheat cultivars. J Radioanal Nucl Chem 307:1055–1063
Field A (2013) Discovering statistics using IBM SPSS statistics. Sage Publications Ltd, London
Foehse D, Jungk A (1983) Influence of phosphate and nitrate supply on root hair formation of rape, spinach and tomato plants. Plant Soil 74:359–368
Gahoonia TS, Nielsen NE (2003) Phosphorus (P) uptake and growth of a root hairless barley mutant (bald root barley, brb) and wild type in low- and high-P soils. Plant Cell Environ 26:1759–1766
Gahoonia TS, Nielsen NE (2004) Barley genotypes with long root hairs sustain high grain yields in low-P field. Plant Soil 262:55–62
Gahoonia TS, Care D, Nielsen NE (1997) Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant Soil 191:181–188
Galkovskyi T, Mileyko Y, Bucksch A, Moore B, Symonova O, Price CA, Topp CN, Iyer-Pascuzzi AS, Zurek PR, Fang SQ, Harer J, Benfey PN, Weitz JS (2012) GiA roots: software for the high throughput analysis of plant root system architecture. BMC Plant Biol 12:116
Gaume A, Mächler F, De León C, Narro L, Frossard E (2001) Low-P tolerance by maize (Zea mays L.) genotypes: significance of root growth, and organic acids and acid phosphatase root exudation. Plant Soil 228:253–264
Haling RE, Brown LK, Bengough AG, Young IM, Hallett PD, White PJ, George TS (2013) Root hairs improve root penetration, rootsoil contact, and phosphorus acquisition in soils of different strength. J Exp Bot 64:3711–3721
Hill JO, Simpson RJ, Ryan MH, Chapman DF (2010) Root hair morphology and mycorrhizal colonisation of pasture species in response to phosphorus and nitrogen nutrition. Crop Pasture Sci 61:122–131
Horn R, Wingen LU, Snape JW, Dolan L (2016) Mapping of quantitative trait loci for root hair length in wheat identifies loci that co-locate with loci for yield components. J Exp Bot 67:4535–4543
Horst WJ, Abdou M, Wiesler F (1993) Genotypic differences in phosphorus efficiency of wheat. Plant Soil 155:293–296
Hu YF, Ye XS, Shi L, Duan HY, Xu FS (2010) Genotypic differences in root morphology and phosphorus uptake kinetics in Brassica napus under low phosphorus supply. J Plant Nutr 33:889–901
Iyer-Pascuzzi AS, Symonova O, Mileyko Y, Hao YL, Belcher H, Harer J, Weitz JS, Benfey PN (2010) Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiol 152:1148–1157
James RA, Weligama C, Verbyla K, Ryan PR, Rebetzke GJ, Rattey A, Richardson AE, Delhaize E (2016) Rhizosheaths on wheat grown in acid soils: phosphorus acquisition efficiency and genetic control. J Exp Bot 67:3709–3718
Jones GPD, Blair GJ, Jessop RS (1989) Phosphorus efficiency in wheat—a useful selection criterion? Field Crops Res 21:257–264
Kenobi K, Atkinson JA, Wells DM, Gaju O, De Silva JG, Foulkes MJ, Dryden IL, Wood ATA, Bennett MJ (2017) Linear discriminant analysis reveals differences in root architecture in wheat seedlings related to nitrogen uptake efficiency. J Exp Bot 68:4969–4981
Keyes SD, Daly KR, Gostling NJ, Jones DL, Talboys P, Pinzer BR, Boardman R, Sinclair I, Marchant A, Roose T (2013) High resolution synchrotron imaging of wheat root hairs growing in soil and image based modelling of phosphate uptake. New Phytol 198:1023–1029
Kono Y, Yamauchi A, Nonoyama T, Tatsumi J, Kawamura N (1987) A revised experimental system of root-soil interaction for laboratory worker. Environ Control Biol 25:141–151
Kuijken RCP, Van Eeuwijk FA, Marcelis LFM, Bouwmeester HJ (2015) Root phenotyping: from component trait in the lab to breeding. J Exp Bot 66:5389–5401
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713
Liu Q (2015) Regional difference of NPK fertilizers application and environmental risk assessment in Jiangsu Province, China. J Appl Ecol 26:1477–1483
Lobet G, Pound MP, Diener J, Pradal C, Draye X, Godin C, Javaux M, Leitner D, Meunier F, Nacry P, Pridmore TP, Schnepf A (2015) Root system markup language: toward a unified root architecture description language. Plant Physiol 167:617–627
Lynch J (1995) Root architecture and plant productivity. Plant Physiol 109:7–13
Lynch JP (2007) Roots of the second green revolution. Aust J Bot 55:493–512
Lynch JP (2011) Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156:1041–1049
Lynch JP, Brown KM (2001) Topsoil foraging—an architectural adaptation of plants to low phosphorus availability. Plant Soil 237:225–237
Ma Z, Bielenberg DG, Brown KM, Lynch JP (2001) Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ 24:459–467
Mcdonald G, Bovill W, Taylor J, Wheeler R (2015) Responses to phosphorus among wheat genotypes. Crop Pasture Sci 66:430–444
Meister R, Rajani MS, Ruzicka D, Schachtman DP (2014) Challenges of modifying root traits in crops for agriculture. Trends Plant Sci 19:779–788
Miguel MA, Postma JA, Lynch JP (2015) Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiol 167:1430-U504
Mooney SJ, Pridmore TP, Helliwell J, Bennett MJ (2012) Developing X-ray computed tomography to non-invasively image 3-D root systems architecture in soil. Plant Soil 352:1–22
Mori A, Fukuda T, Vejchasarn P, Nestler J, Pariasca-Tanaka J, Wissuwa M (2016) The role of root size versus root efficiency in phosphorus acquisition of rice. J Exp Bot 67:1179–1189
Neto AP, Favarin JL, Hammond JP, Tezotto T, Couto HTZ (2016) Analysis of phosphorus use efficiency traits in Coffea genotypes reveals Coffea arabica and Coffea canephora have contrasting phosphorus uptake and utilization efficiencies. Front Plant Sci 7:1–10
Niu YF, Chai RS, Jin GL, Wang H, Tang CX, Zhang YS (2013) Responses of root architecture development to low phosphorus availability: a review. Ann Bot 112:391–408
Osborne LD, Rengel Z (2002) Genotypic differences in wheat for uptake and utilisation of P from iron phosphate. Aust J Agric Res 53:837–844
Paez-Garcia A, Motes C, Scheible W-R, Chen R, Blancaflor E, Monteros M (2015) Root traits and phenotyping strategies for plant improvement. Plants 4:334–355
Roberts TL, Johnston AE (2015) Phosphorus use efficiency and management in agriculture. Resour Conserv Recycl 105:275–281
Shenoy VV, Kalagudi GM (2005) Enhancing plant phosphorus use efficiency for sustainable cropping. Biotechnol Adv 23:501–513
Shepherd JG, Kleemann R, Bahri-Esfahani J, Hudek L, Suriyagoda L, Vandamme E, Van Dijk KC (2016) The future of phosphorus in our hands. Nutr Cycl Agroecosyst 104:281–287
Sinha SK, Rani M, Kumar A, Kumar S, Venkatesh K, Mandal PK (2018) Natural variation in root system architecture in diverse wheat genotypes grown under different nitrate conditions and root growth media. Theor Exp Plant Physiol 30:223–234
Steingrobe B, Schmid H, Claassen N (2001) Root production and root mortality of winter barley and its implication with regard to phosphate acquisition. Plant Soil 237:239–248
Topp CN, Bray AL, Ellis NA, Liu ZB (2016) How can we harness quantitative genetic variation in crop root systems for agricultural improvement? J Integr Plant Biol 58:213–225
Van Dusschoten D, Metzner R, Kochs J, Postma JA, Pflugfelder D, Buhler J, Schurr U, Jahnke S (2016) Quantitative 3D analysis of plant roots growing in soil using magnetic resonance imaging. Plant Physiol 170:1176–1188
Vance CP, Uhde-Stone C, Allan DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol 157:423–447
Vandamme E, Renkens M, Pypers P, Smolders E, Vanlauwe B, Merckx R (2013) Root hairs explain P uptake efficiency of soybean genotypes grown in a P-deficient Ferralsol. Plant Soil 369:269–282
Vejchasarn P, Lynch JP, Brown KM (2016) Genetic variability in phosphorus responses of rice root phenotypes. Rice 9:1–16
Wang X, Shen J, Liao H (2010) Acquisition or utilization, which is more critical for enhancing phosphorus efficiency in modern crops? Plant Sci 179:302–306
Wang Y-L, Almvik M, Clarke N, Eich-Greatorex S, Øgaard AF, Krogstad T, Lambers H, Clarke JL (2015) Contrasting responses of root morphology and root-exuded organic acids to low phosphorus availability in three important food crops with divergent root traits. AoB Plants 7:1–11
Wang Y, Thorup-Kristensen K, Jensen LS, Magid J (2016a) Vigorous root growth is a better indicator of early nutrient uptake than root hair traits in spring wheat grown under low fertility. Front Plant Sci 7:1–9
Wang YS, Jensen LS, Magid J (2016b) Differential responses of root and root hair traits of spring wheat genotypes to phosphorus deficiency in solution culture. Plant Soil Environ 62:540–546
Wasaki J, Yamamura T, Shinano T, Osaki M (2003) Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency. Plant Soil 248:129–136
Wheal MS, Fowles TO, Palmer LT (2011) A cost-effective acid digestion method using closed polypropylene tubes for inductively coupled plasma optical emission spectrometry (ICP-OES) analysis of plant essential elements. Anal Methods 3:2854–2863
Wissuwa M (2005) Combining a modelling with a genetic approach in establishing associations between genetic and physiological effects in relation to phosphorus uptake. Plant Soil 269:57–68
York LM, Slack S, Bennett MJ, Foulkes MJ (2018) Wheat shovelomics I: a field phenotyping approach for characterising the structure and function of root systems in tillering species. bioRxiv. https://doi.org/10.1101/280875
Yuan HM, Blackwell M, Mcgrath S, George TS, Granger SJ, Hawkins JMB, Dunham S, Shen JB (2016) Morphological responses of wheat (Triticum aestivum L.) roots to phosphorus supply in two contrasting soils. J Agric Sci 154:98–108
Zhang LT, Li J, Rong TZ, Gao SB, Wu FK, Xu J, Li ML, Cao MJ, Wang J, Hu EL, Liu YX, Lu YL (2014) Large-scale screening maize germplasm for low-phosphorus tolerance using multiple selection criteria. Euphytica 197:435–446
Zhu JM, Kaeppler SM, Lynch JP (2005a) Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor Appl Genet 111:688–695
Zhu JM, Kaeppler SM, Lynch JP (2005b) Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays). Funct Plant Biol 32:749–762
Zhu JM, Mickelson SM, Kaeppler SM, Lynch JP (2006) Detection of quantitative trait loci for seminal root traits in maize (Zea mays L.) seedlings grown under differential phosphorus levels. Theor Appl Genet 113:1–10
Zhu JM, Zhang CC, Lynch JP (2010) The utility of phenotypic plasticity of root hair length for phosphorus acquisition. Funct Plant Biol 37:313–322
Acknowledgements
The authors are grateful to Dr. Glenn McDonald for providing wheat seeds for this research. We greatly appreciate the financial support from Flinders University Research Scholarship (FURS) to the principle author and the GRDC for funding to JS. The authors would like to thank Dr. Emma de Courcy-Ireland and Dr. Georgia Guild for their proofreading.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Sowinski.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nguyen, V.L., Stangoulis, J. Variation in root system architecture and morphology of two wheat genotypes is a predictor of their tolerance to phosphorus deficiency. Acta Physiol Plant 41, 109 (2019). https://doi.org/10.1007/s11738-019-2891-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2891-0