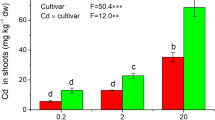
Abstract
Cadmium (Cd), a non-essential and toxic heavy metal element, is extremely harmful for rice growth, food safety and even public health. In this paper, two rice varieties, Shanyou 63 (Tolerant to Cd stress) and Nipponbare (Susceptible to Cd stress), were employed as materials to investigate the protein expression and protein–protein interaction in rice in response to different stages of Cd stress. The result showed that Cd accumulated in the root of both rice cultivars, but the Cd content in the root of Shanyou 63 was significantly lower than that in Nipponbare. Eight proteins were up-regulated in the two rice cultivars, but the expressing abundance of these eight proteins in Shanyou 63 was statistically higher than that in Nipponbare, indicating that the relative abundance of these eight proteins was positively related to the ability of rice cultivars to avoid Cd stress. According to Gene Ontology annotation, four of these eight proteins were involved in energy metabolism, while four participated in stress tolerance. Native-PAGE and LC–MS/MS methods revealed that four GSTs, one cysteine synthase and one protein disulfide isomerase formed a stable protein complex in Shanyou 63 root in response to 3-, 5- and 7-day Cd stresses, but this protein complex did not appear in Nipponbare root under Cd stress. Owing to the same domain, the four glutathione S-transferases (GSTs) and cysteine synthase had the potential to interact and compose a protein complex to detoxify Cd in Shanyou 63 rice plants. Our results provide new insight into the detoxification mechanism of rice plant in response to Cd stress and may prove to be useful for the future research.






Similar content being viewed by others
References
Ahsan N, Lee SH, Lee DG, Lee H, Lee SW, Bahk JD, Lee BH (2007) Physiological and protein profiles alternation of germinating rice seedlings exposed to acute cadmium toxicity. C R Biol 330(10):735–746
Aina R, Labra M, Fumagalli P, Vannini C, Marsoni M, Cucchi U, Bracale M, Sgorbati S, Citterio S (2007) Thiol–peptide level and proteomic changes in response to cadmium toxicity in Oryza sativa L. roots. Environ Exp Bot 59:381–392
Barski OA, Tipparaju SM, Bhatnagar A (2008) The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab Rev 40(4):553–624
Berthold CL, Moussatche P, Richards NG, Lindqvist Y (2005) Structural basis for activation of the thiamin diphosphate-dependent enzyme oxalyl-CoA decarboxylase by adenosine diphosphate. J Biol Chem 280(50):41645–41654
Bertin G, Averbeck D (2006) Cadmium: cellular effects, modifications of biomolecules, modulation of DNA repair and genotoxic consequences (a review). Biochimie 88(11):1549–1559
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brown GK, Otero LJ, LeGris M, Brown RM (1994) Pyruvate dehydrogenase deficiency. J Med Genet 31(11):875–879
Chen X, Lin S, Liu Q, Huang J, Zhang W, Lin J, Wang Y, Ke Y (1844) He H (2014) Expression and interaction of small heat shock proteins (sHsps) in rice in response to heat stress. Biochim Biophys Acta 4:818–828
Chen X, Zhang W, Zhang B, Zhou J, Wang Y, Yang Q, Ke Y, He H (2011) Phosphoproteins regulated by heat stress in rice leaves. Proteome Sci 30(9):37
Cheng F, Zhao N, Xu H, Li Y, Zhang W, Zhu Z, Chen M (2006) Cadmium and lead contamination in japonica rice grains and its variation among the different locations in southeast China. Sci Total Environ 359:156–166
Chepyshko H, Lai CP, Huang LM, Liu JH, Shaw JF (2012) Multifunctionality and diversity of GDSL esterase/lipase gene family in rice (Oryza sativa L. japonica) genome: new insights from bioinformatics analysis. BMC Genom 13:309
Clemens S (2006) Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 88(11):1707–1719
Edwards R, Dixon DP (2005) Plant glutathione transferases. Methods Enzymol 401:169–186
Finn RD, Miller BL, Clements J, Bateman A (2014) iPfam: a database of protein family and domain interactions found in the Protein Data Bank. Nucleic Acids Res 42:D364–D373
Ge CL, Wang ZG, Wan D, Ding Y, Wang Y, Shang Q, Luo SS (2009) Proteomic study for responses to cadmium stress in rice seedlings. Rice Sci 16(1):33–44
Hajduch M, Rakwal R, Agrawal GK, Yonekura M, Pretova A (2001) High-resolution two-dimensional electrophoresis separation of proteins from metal-stressed rice (Oryza sativa L.) leaves: drastic reductions/fragmentation of ribulose-1,5-bisphosphate carboxylase/oxygenase and induction of stress-related proteins. Electrophoresis 22:2824–2831
Hofmann T, Schaefer M, Schultz G, Gudermann T (2002) Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci 99:7461–7466
Hong JK, Choi HW, Hwang IS, Kim DS, Kim NH, Choi DS, Kim YJ, Hwang BK (2008) Function of a novel GDSL-type pepper lipase gene, CaGLIP1, in disease susceptibility and abiotic stress tolerance. Planta 227(3):539–558
Kawashima CG, GoulartC Noji M, Nakamura M, Ogra Y, Suzuki K, Saito K (2004) Heavy metal tolerance of transgenic tobacco plants over-expressing cysteine synthase. Biotechnol Lett 26:153–157
Kuramata M, Masuya S, Takahashi Y, Kitagawa E, Inoue C, Ishikawa S, Youssefian S, Kusano T (2009) Novel cysteine-rich peptides from Digitaria ciliaris and Oryza sativa enhance tolerance to cadmium by limiting its cellular accumulation. Plant Cell Physiol 50(1):106–117
Lee K, Bae DW, Kim SH, Han HJ, Liu X, Park HC, Lim CO, Lee SY, Chung WS (2010) Comparative proteomic analysis of the short-term responses of rice roots and leaves to cadmium. J Plant Physiol 167(3):161–168
Li XJ, Yang MF, Chen H, Qu LQ, Chen F, Shen SH (2010) Abscisic acid pretreatment enhances salt tolerance of rice seedlings: proteomic evidence. Biochim Biophys Acta 1804(4):929–940
Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Jackson JD, Ke Z, Lanczycki CJ, Lu F, Marchler GH, Mullokandov M, Omelchenko MV, Robertson CL, Song JS, Thanki N, Yamashita RA, Zhang D, Zhang N, Zheng C, Bryant SH (2011) CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res 39:D225–D229
Matsuda T, Kuramata M, Takahashi Y, Kitagawa E, Youssefian S, Kusano T (2009) A novel plant cysteine-rich peptide family conferring cadmium tolerance to yeast and plants. Plant Signal Behav 4(5):419–421
Moons A (2003) OsGSTU3 and OsGSTU4, encoding tau class glutathione S-transferases, are heavy metal and hypoxic stress-induced and differentially salt stress-responsive in rice roots. FEBS Lett 553:427–432
Musrati RA, Kollárová M, Mernik N, Mikulásová D (1998) Malate dehydrogenase: distribution, function and properties. Gen Physiol Biophys 17(3):193–210
Nakamoto RK, Baylis Scanlon JA, Al-Shawi MK (2008) The rotary mechanism of the ATP synthase. Arch Biochem Biophys 476(1):43–50
Naranjo MA, Forment J, Roldán M, Serrano R, Vicente O (2006) Overexpression of Arabidopsis thaliana LTL1, a salt-induced gene encoding a GDSL-motif lipase, increases salt tolerance in yeast and transgenic plants. Plant Cell Environ 29(10):1890–1900
Narberhaus F (2002) α-Crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev 66:64–93
Ogo Y, Kakei Y, Itai RN, Kobayashi T, Nakanishi H, Takahashi H, Nakazono M, Nishizawa NK (2014) Spatial transcriptomes of iron-deficient and cadmium-stressed rice. New Phytol 201(3):781–794
Oh IS, Park AR, Bae MS, Kwon SJ, Kim YS, Lee JE, Kang NY, Lee S, Cheong H, Park OK (2005) Secretome analysis reveals an Arabidopsis lipase involved in defense against Alternaria brassicicola. Plant Cell 17(10):2832–2847
Singh KK, Ghosh S (2013) Regulation of glutamine synthetase isoforms in two differentially drought-tolerant rice (Oryza sativa L.) cultivars under water deficit conditions. Plant Cell Rep 32(2):183–193
Sun L, Zheng M, Liu H, Peng S, Huang J, Cui K, Nie L (2014) Water management practices affect arsenic and cadmium accumulation in rice grains. Sci World J 2014:596438
Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa NK, Nakanishi H (2012) The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ 35(11):1948–1957
Tang M, Mao D, Xu L, Li D, Song S, Chen C (2014) Integrated analysis of miRNA and mRNA expression profiles in response to Cd exposure in rice seedlings. BMC Genom 15(1):835
Ueno D, Yamaji N, Kono I, Huang C, Ando T, Yano M, Ma J (2010) Gene limiting cadmium accumulation in rice. PNAS 107(38):16500–16505
Venkatachalam K, Montell C (2007) TRP channels. Annu Rev Biochem 76:387–417
Wang F, Wang Z, Zhu C (2012) Heteroexpression of the wheat phytochelatin synthase gene (TaPCS1) in rice enhances cadmium sensitivity. Acta Biochim Biophys Sin 4(10):886–893
Watanabe T, Shimbo S, Nakatsuka H, Koizumi A, Higashikawa K, Matsuda-Inoguchi N, Ikeda M (2004) Gender-related difference, geographical variation and time trend in dietary cadmium intake in Japan. Sci Total Environ 329:17–27
Wilkinson B, Gilbert HF (2004) Protein disulfide isomerase. Biochim Biophys Acta 1699(1–2):35–44
Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10(9):1539–1550
Xue D, Jiang H, Deng X, Zhang X, Wang H, Xu X, Hu J, Zeng D, Guo L, Qian Q (2014) Comparative proteomic analysis provides new insights into cadmium accumulation in rice grain under cadmium stress. J Hazard Mater 280:269–278
Zhang CF, Peng SB, Peng XX (1997) Response of glutamine synthetase isoforms to nitrogen sources in rice (Oryza Sativa L.) roots. Plant Sci 125:163–170
Acknowledgments
We thank the anonymous referee whose constructive comments were very helpful in improving the quality of this work. This work was supported by the Natural Science Foundation of China and Fujian (Grant Nos. 31270454, 61163047, 81502091 and 2013J01077), a grant from the Education Department of Fujian (Grant No. JA10103) and the Key Program of Ecology in Fujian (Grant Nos. 0608507 and 0b08b005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. Hajduch.
S. Lin, W. Wan and T. Tian contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, S., Wan, W., Tian, T. et al. Protein complex and proteomic profile in the roots of Oryza sativa L. in response to cadmium toxicity. Acta Physiol Plant 37, 188 (2015). https://doi.org/10.1007/s11738-015-1936-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1936-2