Abstract
Key message
Zn caused an increase of photosynthesis activity, carbohydrates and chlorophyll at the level 1–2.5 mM, while phenols revealed a continuous increase together with Zn increase in the medium.
Abstract
The aim of the present study was to determine the effect of various Zn application levels on some physiological, morphological and biochemical parameters of a hybrid Salix purpurea × triandra × viminalis 2. Plants were cultivated under control conditions with application of Zn in the range 0–5 mM. The effects on net photosynthesis rate (P N), stomatal conductance (g s), transpiration rate (E), intercellular CO2 (C i), chlorophyll a and b, carotenoids, growth, sugars and phenols were analysed. Our investigations confirmed a dual role of Zn, with benefits at the level 1–2.5 mM, and a decrease of measured photosynthesis activity, carbohydrates and chlorophyll below and above this range. Moreover, the decrease of these parameters at the highest Zn application did not reach the level of control plants. This means that this species may have further potential as an accumulator in polluted areas. However, phenols revealed a continuous increase together with Zn increase in the medium.
Similar content being viewed by others
Introduction
Recently, there has been observed increasing concern about Zn concentration in the soil and water (Zarcinas et al. 2004) due to natural and human activities, such as mining and industrial processes, as well as agricultural practices—use of fertilizers containing various heavy metals (Dudka et al. 1996). On the other hand, there has also been reported a deficiency of this element in agricultural areas of India (Singh 2009). Zinc is one of the trace elements with a dual role in the environment. Its high role in plant metabolic processes is already known. This element activates enzymes and is involved in protein synthesis involved in plant metabolism (nitrogen metabolism, photosynthesis and auxin synthesis) and in carbohydrate, nucleic acid and lipid metabolism. It is the only metal represented in all six enzyme classes (Broadley et al. 2007). At appropriate concentrations, zinc increases plant productivity, growth and development of fruits (Vaillant et al. 2005). The beneficial role of zinc is limited due to its concentration in the environment, as well as in plants, and its level is quite narrow: 15–100 ppm (Clemens 2006). This element can be easily assimilated by plants from contaminated soils, exceeding the required level for plant nutrition. Plants affected by excessive levels of zinc exhibited similar symptoms as for other heavy metals. The effects included chlorosis, inhibited germination, stunted growth, reduced leaf number and area, reduced yield and reduced flower production. These symptoms are directly connected with plant functioning: such processes as photosynthesis, dysfunction of relevant enzymes, impairment of nutrient uptake, plant wilting and altered water relations (Prasad 2004; Deng et al. 2006; Dhir et al. 2008). Excess zinc can cause an imbalance in proper functioning of transpiration, photosynthesis and photosynthetic electron transport, as well as biosynthesis of chlorophyll and cell membrane integrity (Richardson et al. 1993). This might be connected with formation of reactive oxygen species (ROS), which can affect photosynthetic activity as well as further plant growth (Cuypers et al. 2001). Photosynthesis is affected by damage of photosynthetic apparatus, inhibition of electron transport, carbon fixation capacity and photophosphorylation potential (Küpper et al. 1996). It was found that especially photosystem II (PS II) is affected by an excessive level of Zn (Vernay et al. 2007; Paiva et al. 2009; Janik et al. 2010; Dhir et al. 2011), while on the other hand an increase of PS I activity supported the build-up of a transthylakoid proton gradient, which subsequently helped maintain the photophosphorylation potential (Dhir et al. 2011). Previous investigations revealed a decrease of photosynthesis rate, due to a large reduction of stomatal conductance and the transpiration rate (Mukhopadhyay et al. 2013), which might be connected with the effect of Zn excess on the photosynthetic apparatus (Prasad and Strzalka 1999). Moreover, Zn could cause decreased activity of many chloroplastic and peroxisomal enzymes (such as RuBPC/O) and in turn a decrease of photosynthesis activity (van Assche et al. 1980).
One of the visible symptoms of Zn excess can be chlorosis, because of the possibility of displacing other metals, such as Fe and Mg, due to a similar ion radius (Marschner 1995), leading to metal-substituted chlorophylls of reduced functionality (Küpper et al. 1996). Decline of photosynthetic pigments might also be caused by inhibition of the pigment’s synthesis (Prasad and Prasad 1987) or direct oxidative damage of the pigments (Oláh et al. 2010). As an effect of Zn deficiency and excess, a decrease of chlorophyll a and b was recorded, while a decrease of carotenoids was noted in the case of an excessive Zn level. Together with a high Zn level, chloroplast damage was observed, such as membrane rupture and thylakoid disintegration, which subsequently could affect the process of photosynthesis (Mukhopadhyay et al. 2013). Determination of chlorophylls is an important parameter in ecophysiological research. Chlorophyll content is a useful estimation of primary productivity and has been used in bioassays of environmental stresses (Makinde and Akande 2012). It provides valuable information about the physiological status of plants and can indicate the occurrence of metal intoxication. Moreover, it indicates indirectly the nutritional status of plants since much of leaf nitrogen is incorporated in chlorophyll (Moran et al. 2000). Another group of pigments, the carotenoids, plays a structural role in the organization of photosynthetic membranes, participation in light harvesting, energy transfer, interception of deleterious free oxygen and organic radicals and quenching (Dobroviczká et al. 2013). Therefore, both leaf pigment contents can be used as an indicator of metal toxicity.
The decrease of plant growth might be a consequence of the effect of Zn excess on photosynthesis as well as ROS production. In Zn-deprived leaves, vacuoles were characterised by electron dense material resembling phenolic compounds, and a phenol assay revealed elevated content of phenolic compounds (Mukhopadhyay et al. 2013). This was true for deficiency and excess of Zn (Kim and Wetzstein 2003). Phenolics are wildly distributed secondary metabolites, enhanced biosynthesis of which is observed under metal stress (Michalak 2006). They act as antioxidants due to the availability of their hydroxyls, and the number of hydroxyl groups in the structure affects antioxidant activity (Kováčik and Klejdus 2008). Additionally, phenolics can also chelate metals (Michalak 2006).
The aim of the study was to examine selected physiological, morphological and biochemical aspects of hybrid Salix purpurea × triandra × viminalis 2 response to various Zn levels. This work is a continuation and development of our previous studies (in press) about phytoextraction of Zn in selected organs and biomass of the tested hybrid, which is the most promising willow taxon within our whole collection. The presented data are an extension of numerous experiments performed in polluted areas in 2011–2013 in Poland. All our Salix taxa were verified as regards their abilities to use diverse aspects such as phytoextraction of trace elements, biomass and raw material, a substrate for the wood industry, the chemical industry and medicine. For this reason, the whole tested population was divided and the most promising plants were tested.
Materials and methods
Experimental design
This work is a continuation of our studies described by Goliński et al. (2015), based on the same hydroponic experiment and experimental materials (2-year-old standardized rods of S. purpurea × triandra × viminalis 2 taxon collected from 2-year-old rootstock). In experiment, the modified Knop medium prepared according to Barabasz et al. (2010)’s methodology was used to grow Salix taxon in solutions without (control system—c) and with diverse addition of Zn: 0.1, 0.2, 1.0, 2.5 and 5.0 mMof Zn in Zn(NO3)2 6H2O form. Electrolytic conduction of the initial solution was 1.63 mS/cm (PN-ISO 1265 + AC1:1997), while pH was 5.8 (PN-ISO 10390:1997). One Salix cuttings was grown in one pot and in 6 replicates for each solution with concrete Zn addition. The pH of individual solutions was controlled using the automatic addition of 1 M HCl or 1 M KOH. This experiment lasted 21 days and was performed under controlled conditions (phytotron): relative humidity of 72 ± 4 %, temperature from 21 to 16 ± 1 °C (day/night) and a 16-h photoperiod.
Photosynthetic activity investigations
Matured leaves were selected for measurements of photosynthetic activity. Four photosynthetic intensity parameters, such as net photosynthesis (P N), stomatal conductance (g s), C i intercellular CO2 concentration and transpiration rate (E), were analysed with using the handheld photosynthetic system Ci 340aa (CID Biosciences Inc., Camas, USA). The stable conditions of investigations in the leaf chamber were provided: CO2 inflow concentration [390 µmol (CO2) mol−1], photosynthetic photon flux density (PPFD) 1000 µmol (photon) m−2 s−1, chamber temperature 25 °C and relative humidity 40 ± 3 %. Investigations were performed between 10:00 and 15:00 h. Water use efficiency of photosynthesis (WUEph) at leaf level was analysed as the relationship between net CO2 exchange and transpiration from measurements of leaf gas exchange (Erice et al. 2011).
Chlorophyll content
Based on Hiscox and Israelstam (1979), 5 cm3 of DMSO was poured onto half-gram samples of willow leaves without petioles, which were then incubated at 65 °C for one hour. After cooling, samples were 20-fold (DMSO) diluted and chlorophyll (Chl) and carotenoid (Car) content were determined spectrophotometrically (UV–VIS spectrophotometer, Jasco V 530) at three wavelengths (480, 649 and 655 nm). The pigment concentrations were read using the Jasco—Quantitative Analysis computer program. The contents of Car, Chl a, Chl b and total Chl a + b were calculated according to the modified Arnon’s formulas (Wellburn 1994) and expressed in milligrams of fresh weight of tissue.
Extraction of phenolic compounds
The willow leaves were grounded in liquid nitrogen to a fine powder and approximately 0.5 g was mixed with 10 ml of 80 % methanol. Samples were sonicated, then stirred for 1 h and centrifuged. The extraction was repeated twice. Both supernatants were mixed and stored at a cold temperature until the analyses.
Total phenolic content
The total phenolic content of was measured using Folin-Ciocalteu’s method with some modifications (Dewanto et al. 2002). The standard solution and the extract were mixed with 0.1 ml of Folin-Ciocalteu’s reagent. After 3 min, 1 ml of 10 % Na2CO2 solution was added. The absorbance at λ = 765 nm was measured with a Cary 300 Bio UV–Vis spectrophotometer by Varian after 30-min incubation at room temperature. The total phenolic content was expressed as milligrams of gallic acid per gram of dry extract.
Total flavonoid content
Total flavonoid content was measured according to Lin and Tang (2007) and Choi et al. (2006) with some modifications. 250 µl of methanolic extract was mixed with 1.25 ml of distilled water and 75 µl of 5 % NaNO2. After 6 min, 150 µl of 10 % AlCl3 and after the further 6 min 500 μl of 1 M NaOH and 275 μl of distilled water were added to the mixture. The absorbance was measured at 510 nm, and the total flavonoid content was expressed as mg rutin equivalents per g of dried extract.
Chromatographic analysis
Prior to analysis, dried methanolic extracts of leaf samples were dissolved in 1 ml of methanol. HPLC analysis was performed using a Waters Alliance 2695 Chromatograph coupled with a Waters 2996 Photodiode Array Detector. Chromatographic separation was performed on an RP C-18 column, 250 × 4 mm × 5 μm, with an acetonitrile: 2 % aqueous acetic acid mixture (pH 2) used as an elution phase (gradient). Concentration of phenolic acids was determined using an internal standard at the wavelength λ = 280 nm (gallic, p-hydroxybenzoic, p-coumaric acids) or λ = 320 and 280 nm (ferulic and t-cinnamic acids), myricetin (C15H10O8) λ = 320 nm. Compounds were identified based on a comparison of retention time of the analysed peak with the retention time of the standard and by adding a specific amount of the standard to the analysed samples and a repeated analysis. Detection level was 1 μg g−1.
Soluble carbohydrates
The sugars were extracted from samples of willow leaf of approximately 0.5 g for 60 min with 10 ml of ethanol/water mixture (80:20, v/v) at 80 °C (Johansen et al. 1996). The extract was filtered through AP 200 1300 glass fibre pre-filters (Millipore). After solvent evaporation, 1 ml of mobile phase (acetonitrile/water, 75:25, v/v) was added and the extracts were analysed with a Waters Alliance 2695 Chromatograph coupled with a Waters RI detector 2414. The acetonitrile/water mobile phase (75:25, v/v) was used at a flow rate of 1.0 ml min−1 to separate soluble sugars (fructose, glucose and sucrose) on a Supelcosil LC NH2 column.
Morphological parameters
Analysis of number and length of shoots was performed in each 21-day experiment, while the determination of leaf area and their total amount was done on the 1st and 21st days of the experiment only. The total leaf area was measured with a DOCUPEN RC 800 portable scanner with ABBYY FineReader 6.0 Sprint and Adobe Photoshop 9.0 software.
Statistical analysis
The data were analysed with the statistical software STATISTICA 9.1. Results were analysed with a factorial ANOVA with “Zn level” and “day of measurement” as fixed factors. Tukey’s test was employed to analyse differences between measured parameters. A graphical presentation of Tukey’s test results is provided in the present study. For determination of structure and rules in relations between variables, principal component analysis (PCA) was used. In this analysis, the orthogonal transformation of observed variables to a new set of non-correlated variables (components) was performed.
Results
One-way analysis of variance revealed a significant influence of Zn level on all photosynthetic activity parameters. The highest level of net photosynthesis rate was recorded for plants cultivated with Zn application at the level of 0.2 and 1.0 mM. The lowest level was noted for control plants. Plants treated at the highest Zn level showed a decrease of P N but did not reach the level of the control. Stomatal conductance was the highest for plants cultivated under 1.0 mM of Zn, and this time this parameter was affected by the highest concentration of Zn and a decline below the control level. The opposite response was noted for intercellular CO2 concentration; hence, the lowest levels were noted for 0.1, 0.2 and 1.0 mM Zn concentrations, and the highest for 5.0. Transpiration rate revealed the highest level for 1.0 mM Zn and the lowest for control. Similarly as for P N, a decrease was noted at 2.5 and 5.0 mM, though not so high as for the control. Water use efficiency was at similar level for control and 0.1 and 0.2 mM of zinc. The lowest level was observed for 1.0 mM of Zinc, while an increase was observed for higher concentrations (Fig. 1).
Mean ± SE of P N net photosynthesis rate (a), C i intercellular CO2 concentration (b), g s stomatal conductance (c), E transpiration rate (d) and WUEph water use efficiency (e) in Salix leaves at different Zn application levels. Different letters denote significant differences at level α = 0.05; F statistics of one-way ANOVA with Zn level influence factor (**α ≤ 0.01; ***α ≤ 0.001)
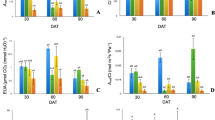
Chlorophyll content was the highest for plants treated with 2.5 mM Zn. Similarly as for photosynthesis rate, a decrease was recorded for plants treated with 5 mM Zn, but not as high as for the control or 0.1 mM Zn. A similar pattern was noted for carotenoids (Fig. 2).
One-way analysis of variance revealed a statistically significant effect of Zn application level of glucose, fructose and sucrose in Salix purpurea × triandra × viminalis 2 leaves. All three carbohydrates showed an increase together with Zn level in the medium. The glucose and sucrose content rose from 23.033 (control) to 37.80 mg g−1 DW (5 mM of Zn) and from 18.018 mg g−1 DW (control) to 35.28 mg g−1 DW (5 mM of Zn), respectively. The fructose content increased from 23.79 (control) to 37.08 mg g−1 DW (5 mM of Zn); however, a similar level was recorded for plants with the three highest Zn applications (Fig. 3).
Growth parameters showed a different pattern of response. Two-way ANOVA revealed a significant effect (α ≤ 0.05) of Zn level on all measured plant morphological features (Table 1). The highest shoot length (134.03 ± 8.17 cm) was noted for plants treated with Zn at the level 2.5, while the lowest was noted for 1.0 and 5.0 mM Zn (72.80 ± 10.42 and 72.87 ± 29.41 cm, respectively). A similar pattern, although not always statistically significant, was noted for number of shoots, leaves and leaf area (Fig. 4).
Total phenol content significantly (α ≤ 0.05) increased together with Zn level in the medium. For control, the content of phenolics was 9.516 ± 0.244, while at 5 mM of Zn it was 20.924 ± 0.099 mg g−1 GAE DW. Flavonoids also increased in comparison to the control from 4.148 ± 0.152 to 9.974e ± 0.396 mg g−1 rutin DW, but especially so between 2.5 and 5.0 mM Zn levels. In the control, both phenolic acids (trans-cinnamic acids and their derivatives: chlorogenic, p-coumaric and ferulic and derivatives of benzoic acids: vanillic and synaptic) and flavonoids (myricetin and quercetin) were found. Ferulic acid even showed a decrease for the two lowest Zn application levels, while a significant increase was noted for the two highest Zn levels. The concentration of chlorogenic and vanillic acids did not change under Zn application. Sinapic acid showed a slow increase together with Zn increase in the medium. The p-coumaric acid concentration only for 0.2 and 2.5 mM of Zn was significantly different than in the control. Trans-cinnamic, myricetin and quercetin showed a dynamic, significant increase (Table 2).
Principal component analysis revealed a negative relation between P N, g s and Zn accumulation in leaves, while a positive relation was noted for C i and Zn accumulation in leaves and shoots. Chlorophyll and carotenoids were found as positively related to P N, E, g s and Zn in shoots and leaves. Similar relations to photosynthetic activity and Zn accumulation in plant organs were observed for carbohydrates. A slightly negative relation of morphological parameters and photosynthetic activity parameters and Zn accumulation was observed. For phenol relations, only total phenols and flavonoids were related due to similar tendencies of all of them. A positive relation was found between phenols, flavonoids and Zn accumulation in leaves and shoots, as well as with intercellular CO2 concentrations. The lack of a relation or a slightly negative relation of above-mentioned parameters was noted for P N, E and g s (Fig. 5).
Discussion
Zinc is known for its importance for proper plant functioning. Hence, some amount of this element is necessary, while exceeding this optimum might negatively influence plants. On the other hand, there is a group of plants with higher tolerance to zinc in the environment, which can be used in highly polluted areas Malva neglecta, Salvia spinosa (Nematian and Kazemeini 2013), Thlaspi caerulescens (Taiz and Zeiger 2010), Corydalis pterygopetala Franch or Sonchus asper (L.) Hill (Yanqun et al. 2005). Zinc is also an important element for the process of photosynthesis, the photosynthetic apparatus and pigments. However, high levels can cause an imbalance of this process, due to disturbance of stomatal opening and in consequence photosynthesis intensity. There was also found a specific effect on the Calvin cycle (Chaney 1993) and photosystem activities (van Assche and Clijsters 1986). The disturbances of g s and P N can cause higher accumulation of CO2. Our investigations confirmed the beneficial role of zinc at a certain level, while deficiency and excess of this element caused a decrease of photosynthesis intensity parameters. This was also shown in research on tea plantlets (Mukhopadhyay et al. 2013). However, the decrease in P N for 5 mM Zn was not so high, as Fernandez et al. (2012) noted in their investigations with two poplar clones. They even found a decrease of P N, g s and E for 1 mM in comparison to control. Similar results were also obtained in four Datura species, where similar Zn solutions were applied, and they showed a decrease of P N and g s, while C i increased together with Zn increase in the medium (Vaillant et al. 2005).
On the other hand, the beneficial role of Zn for plants was also observed in relation to other heavy metals; e.g. Ali et al. (2000) found that Zn can improve photosynthesis and stomatal conductance under Cd stress. However, they did not observe any changes of P N and g s of plants cultivated without Cd and an increasing Zn level. Our investigations also revealed the beneficial role of Zn on water exchange, with an increase for 1 mM Zn application, while inhibition was observed below and above this level. It was previously found that Zn excess can affect water use efficiency and in consequence merely act on chloroplast functions (Clijsters and van Assche 1985). It was also found that Zn plays a structural role of cell membrane component, and in conditions of Zn deficiency a cell membrane integrity can be noted, and as a result higher membrane permeability can be observed (Weich et al. 1982). Zn plays an important role to protect lipids from membrane damage and proteins from peroxidation caused by reactive oxygen species (ROS) (Alloway 2008) and furthermore in protection against water losses. The beneficial role of small concentrations of zinc on WUE was also observed in our investigations, while higher concentrations caused sudden decrease of WUE and did not reach the level comparable to the control. This probably can also cause a negative effect on photosynthesis intensity. Both photosynthesis and transpiration, in response to excess of zinc, are related to stomatal closure. Zn was found to inhibit net photosynthesis by increasing stomatal closure and mesophyll resistance to CO2 uptake (Prasad and Strzalka 1999). It was also discovered that Zn stress inhibited K+ uptake, and decrease of stomatal conductance may be related to an alternation in the K+/Ca2+ ratio in the guard cells and/or to the abscisic acid concentration, which controls the stomatal movement (Marschner 1995).
Many studies have proved that photosynthetic pigment content may reflect plant sensitivity to stress conditions, including heavy metals. Contrary to many studies, in Zn-treated willow plants higher chlorophyll (Chl) accumulation than in controls was observed. Reduced Chl content in different plant species has been well documented (Prasad and Strzalka 1999; Mishra and Dubey 2005; Aggarwal et al. 2012), suggesting that heavy metal ions could interfere with Chl biosynthesis either through central Mg ion substitution or through direct inhibition of enzymatic steps (Cenkci et al. 2010; Pourraut et al. 2011). The increase in Chl content in willow leaves may be regarded as an adaptive mechanism to toxic conditions. This indicates that the primary metabolism is not subject to change. Probably, it could be preserved by the mechanisms leading to the accumulation of metal chelate in the vacuole. Pigment content in plants is characteristic for each species and even cultivar. Chl accumulation in Zn2+ treatment plants depends on the individual tolerance toward zinc toxicity. There are a few reports that have shown the enhancement of pigments after exposure to metals (Mishra and Dubey 2005). We also observed a slight increase of Chl a + b, as in the case of barley leaves that were grown in the presence of Zn for 7 days (Stiborova et al. 1986). Moreover, the chlorophyll ratio (Chl a/b), which is used as a stress indicator, was close to the control. Usually, in plants exposed to high concentrations of metals this ratio (Chl a/b) decreases (Krzesłowska et al. 2010). In our studies, it increased slightly, only in stressed plants, together with enhancing concentrations of Zn ions, similarly to bean seedlings (Phaseolus vulgaris) under different Cd, Cu and Pb doses (Zengin and Munzuroglu 2005) and to black crowberry (Empetrum nigrum) leaves near a Cu and Ni smelter in the field (Monni et al. 2001).
Also carotenoids are involved in several aspects of photosynthesis. They protect chloroplasts from excess energy. As antioxidants they defend against photooxidative damage, by quenching singlet-state chlorophyll and ROS and inhibiting lipid peroxidation under stress conditions (Kenneth et al. 2000; Hou et al. 2007; Sengar et al. 2008). The carotenoid content increased with the increase in concentration of Zn ions, indicating cell protection against oxidative changes, similarly as in the previous studies on other species (Tewari et al. 2002). We also observed an increase in chlorophyll/carotenoid ratio (Chl/Car). This ratio is analysed as an indicator of the specificity of metal interaction with pigments, and its value depends on the type of metal and the growth stage of plants used in experiments (Myśliwa-Kurdziel and Strzałka 2002). After two-week copper treatment of 4-week-old spinach and oat plants, a slow rise in this ratio was observed (Baszyński et al. 1988). Also nickel applied to etiolated bean during greening increased the Chl/Car ratio (Krupa et al. 1993). Although the authors of these studies suggest that this is evidence of the slightly stronger inhibition of Car accumulation than that of Chl, in our research carotenoid content increased.
The Zn stress induced increases of glucose, fructose and sucrose in leaves of Salix. In regular plant metabolism, assimilates are produced in leaves, then transferred to different tissues and stored in response to sink–source relations regulated by partitioning of starch and sucrose biosynthesis (Taiz and Zeiger 2006). Generally, metal stress caused changes in carbohydrate concentrations in leaves of plants. The obtained results confirmed our earlier studies concerning the influence of metals (Ni and Cu) on accumulation of soluble sugars in leaves (Drzewiecka et al. 2012; Gąsecka et al. 2012). In leaves of Camellia sinensis, the excess of Zn caused a drop of soluble and reducing sugars (Mukhopadhyay et al. 2013). It was also documented that other metals such as Ni or Cu stimulate accumulation in leaves or needles of Salix sp. and Pinus sylvestris. The enhanced concentration of the sugars in leaves probably is a result of disturbances in starch hydrolysis, as was suggested in earlier studies (Taiz and Zeiger 2006; Drzewiecka et al. 2012). Other studies indicated that the consequences of the accumulation of assimilates were changes in the rate of photosynthesis and disturbances in the sink/source balance within a plant, leading to premature senescence (Wingler et al. 2006; Tholen et al. 2007). In several studies, sugars were postulated to be signalling molecules controlling gene expression and developmental processes in plants (Morkunas et al. 2005; Hanson and Smeekens 2009).
Our results showed increased concentrations of total phenolics, total flavonoids and individual components under Zn stress, pointing to their important role in detoxification mechanisms. Accumulation of phenolics in leaves under metal exposure has also been observed in other studies, e.g. in Salix viminalis under Cu and Ni exposure (Drzewiecka et al. 2012; Gąsecka et al. 2012), in Camellia sinensis under Zn excess (Mukhopadhyay et al. 2013) and in Vaccinium myrtillus near an Zn–Pb smelter (Białońska et al. 2007). Among the phenolic acids, derivatives of t-cinnamic acids, especially chlorogenic acid (Niggeweg et al. 2004), have strong antioxidant activity. Zn did not affect chlorogenic acid and the same was confirmed for vanillic acid. The other phenolics exhibited significantly elevated concentrations under Zn exposure. In other studies, the concentration of phenolic acids changed under exposure to different metals, e.g. in Tillandsia albida the increase of vanillic and caffeic acids under Cd and Cd + Ni stress was observed, while chlorogenic acid concentration increased under Ni exposure but decreased under Cd exposure, and p-coumaric acid did not have an effect on metal concentrations (Kováčik et al. 2012). In the alga Scenedesmus quadricauda under Cu stress conditions, the elevation of all generally detected phenolic acids was documented (Kováčik et al. 2010). Our results suggested that under Zn excess phenolics play an important role in response of Salix plants.
Conclusions
Overall, the investigations revealed the important role of Zn for Salix purpurea × triandra × viminalis 2. Moreover, a decrease of photosynthetic activity parameters at the highest Zn application did not reach the level of control plants. Similar results were noted for chlorophyll, carotenoids and carbohydrates. This means that this taxon can have further potential as an Zn accumulator in polluted areas. Phenols revealed a continuous increase together with Zn increase in the medium.
Author contribution statement
M. M designed the experiment; J. D performed the experiment; K. B performed gas exchange measurements; M. M, M. G, J. D, T. H and T. K performed plant material analyses; K. B performed analyses of experimental data; K. B, M. M, T. H, M. G, P. G, P. R and T. K involved in the preparation of the paper.
References
Aggarwal A, Sharma I, Tripathi BN, Munjal AK, Baunthiyal M, Sharma V (2012) Metal toxicity and photosynthesis in photosynthesis: overviews on recent progress and future perspectives. IK International Publishing House (Pvt) Limited, New Delhi
Ali G, Srivastava PS, Iqbal M (2000) Influence of cadmium and zinc on growth and photosynthesis of Bacopa monniera cultivated in vitro. Biol Plant 43(4):599–601
Alloway BJ (2008) Zinc in soils and crop nutrition, 2nd edn. IZA and IFA, Brussels and Paris
Barabasz A, Krämer U, Hanikenne M, Rudzka J, Antosiewicz DM (2010) Metal accumulation in tobacco expressing Arabidopsis halleri metal hyperaccumulation gene depends on external supply. J Exp Bot 61:3057–3067
Baszyński T, Tukendorf A, Ruszkowska M, Skórzyńska E, Maksymiec W (1988) Characteristics of the photosynthetic apparatus of copper non-tolerant spinach exposed to excess copper. J Plant Physiol 132:708–713
Białońska D, Zobel AM, Kuraś M, Tykarska T, Sawicka-Kapusta K (2007) Phenolic compounds and cell structure in bilberry leaves affected by emissions from a Zn–Pb smelter. Water Air Soil Pollut 181(1–4):123–133
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Cenkci S, Cigerci IH, Yildiz M, Ozay C, Bozdag A, Terzi H (2010) Lead contamination reduces chlorophyll biosynthesis and genome template stability in Brassica rapa L. Environ Exp Bot 67:467–473
Chaney RL (1993) Zinc phytotoxicity. In: Robson AD (ed) Zinc in soils and plants. Kluwer Academic Publishers, Dordrecht, pp 131–150
Choi Y, Lee SM, Chun J, Lee HB, Lee J (2006) Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem 99(2):381–387
Clemens S (2006) Toxic metal accumulation, responses and mechanisms of tolerance in plants. Biochimie 88:1707–1719
Clijsters H, van Assche F (1985) Inhibition of photosynthesis by heavy metals. Photosynth Res 7:31–40
Cuypers A, Vangronsve J, Clijsters H (2001) The redox status of plant cells (AsA and GSH) is sensitive to zinc imposed oxidative stress in roots and primary leaves of Phaseolus vulgaris. Plant Physiol Biochem 39:657–664
Deng H, Ye ZH, Wong MH (2006) Lead and zinc accumulation and tolerance in populations of six wetland plants. Environ Pollut 141:69–80
Dewanto V, Wu X, Liu RH (2002) Processed sweet corn has higher antioxidant activity. J Agric Food Chem 50:4959–4964
Dhir B, Sharmila P, Pardha Saradhi P (2008) Photosynthetic performance of Salvinia natans exposed to chromium and zinc rich wastewater. Braz J Plant Physiol 20:61–70
Dhir B, Sharmila P, Pardha Saradhi P, Sharma S, Kumar R, Mehta D (2011) Heavy metal induced physiological alterations in Salvinia natans. Ecotox Environ Safe 74:1284–1678
Dobroviczká T, Piršelová B, Mészáros P, Blehová A, Iibantová J, Moravčíková J, Matušíková I (2013) Effects of cadmium and arsenic ions on content of photosynthetic Pigments in the leaves of Glycine max (l.) Merrill. Pak J Bot 45(1):105–110
Drzewiecka K, Mleczek M, Gąsecka M, Magdziak Z, Goliński P (2012) Changes in Salix viminalis L. cv. ‘Cannabina’ morphology and physiology in response to nickel ions—hydroponic investigations. J Hazard Mater 217–218:429–438
Dudka S, Piotrowska M, Terelak H (1996) Transfer of cadmium, lead and zinc from industrially contaminated soil to crop: a field study. Environ Pollut 994:181–188
Erice G, Louahliac S, Irigoyenb JJ, Sánchez-Díazb M, Alamid IT, Avicea J-C (2011) Water use efficiency, transpiration and net CO2 exchange of four alfalfa genotypes submitted to progressive drought and subsequent recovery. Environ Exp Bot 72:123–130
Fernandez J, Zacchini M, Fleck I (2012) Photosynthetic and growth responses of Populus clones Eridano and I-214 submitted to elevated Zn concentrations. J Geochem Explor 123:77–86
Gąsecka M, Mleczek M, Drzewiecka K, Magdziak Z, Rissmann I, Hadzinikolau T, Goliński P (2012) Physiological and morphological changes in Salix viminalis L. as a result of plant exposure to copper. J Environ Sci Health A 47(4):548–557
Goliński P, Mleczek M, Magdziak Z, Gąsecka M, Borowiak K, Dąbrowski J, Kaczmarek Z, Rutkowski P (2015) Efficiency of Zn phytoextraction, biomass yield and formation of low-molecular-weight organic acids in S × rubens—a hydroponic experiment. Chem Ecol 31(4):345–364
Hanson J, Smeekens S (2009) Sugars perception and signalling—an update. Curr Opin Plant Biol 12:562–567
Hiscox JD, Israelstam GF (1979) A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot 57:1332–1334
Hou W, Chen X, Song G, Wang Q, Chang C (2007) Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol Bioch 45:62–69
Janik E, Maksymiec W, Mazur R, Garstka M, Gruszecki WI (2010) Structural and functional modifications of the major light-harvesting complex II in cadmium- or copper-treated Secale cereal. Plant Cell Physiol 51:1330–1340
Johansen HN, Glitso V, Knutsen KEB (1996) Influence of extraction solvent and temperature on the quantitative determination of oligosaccharides from plant materials by high-performance liquid chromatography. J Agric Food Chem 44(6):470–1474
Kenneth K, Pallett KE, Young AJ (2000) Carotenoids. In: Ruth GA, Hess JL (eds) Antioxidants in Higher Plants. CRC Press, Boca Raton
Kim T, Wetzstein HY (2003) Cytological and ultrastructural evaluations of zinc deficiency in leaves. J Am Soc Hort Sci 128:171–175
Kováčik J, Klejdus B (2008) Dynamics of phenolic acids and lignin accumulation in metal-treated Matricaria chamomilla roots. Plant Cell Res 27:605–615
Kováčik J, Klejdus B, Hedbavny J, Bačkora M (2010) Effect of copper and salicylic acid on phenolic metabolites and free amino acids in Scenedesmus quadricauda (Chlorophyceae). Plant Sci 178(3):307–311
Kováčik J, Klejdus B, Stork F, Hedbavny J (2012) Physiological responses of Tillandsia albida (Bromeliaceae) to long-term foliar metal application. J Hazard Mat 15:239–240
Krupa Z, Siedlecka A, Maksymiec W, Baszyński T (1993) In vivo response to photosynthetic apparatus of Phaseolus vulgaris L. to nickel toxicity. J Plant Physiol 142:664–668
Krzesłowska M, Samardakiewicz S, Woźny A (2010) Trace metals. In: Woźny A, Goździka-Józefiak A (eds) Cell responses to stress factors. Wydawnictwo Naukowe Uniwersytetu Adama Mickiewicza w Poznaniu, Poznań, pp 90–146 (In Polish)
Küpper H, Küpper F, Spiller M (1996) Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J Exp Bot 47:259–266
Lin JY, Tang CY (2007) Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem 101(1):140–147
Makinde AM, Akande FI (2012) Effects of lead and simulated acid rain on chlorophyll contents of selected tropical mosses. J Sci 14(2):309
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:523–530
Mishra S, Dubey RS (2005) Heavy metal toxicity induced alterations in photosynthetic metabolism in plants. In: Pessarakli M (ed) Handbook of Photosynthesis, 2nd edn. CRC Press, Taylor & Francis Publishing Company, Florida, pp 845–863
Monni S, Uhlig C, Hansen E, Magel E (2001) Ecophysiological responses of Empetrum nigrum to heavy metals pollution. Environ Pollut 112(2):121–129
Moran JA, Mitchell AK, Goodmanson G, Stockburger KA (2000) Differentiation among effects of nitrogen fertilization treatments on conifer seedlings by foliar reflectance: a comparison of methods. Tree Physiol 20:1113–1120
Morkunas I, Marczak Ł, Stachowiak J, Stobiecki M (2005) Sucrose-induced lupine defence against Fusarium oxysporum: sucrose-stimulated accumulation of isoflavonoids as a defence response of lupine to Fusarium oxysporum. Plant Physiol Biochem 43:363–373
Mukhopadhyay MS, Das A, Subba P, Bantawa P, Sarkar B, Ghosh P, Mondal TK (2013) Structural, physiological, and biochemical profiling of tea plants under zinc stress. Biol Plant 57(3):474–480
Myśliwa-Kurdziel B, Strzałka K (2002) Influence of metals on biosynthesis of photosynthetic pigments. In: Prasad MNV, Strzałka K (eds) Physiology and biochemistry of metal toxicity and tolerance in plants. Springer, Netherlands, pp 201–227
Nematian MA, Kazemeini F (2013) Accumulation of Pb, Zn, Cu and Fe in plants and hyperaccumulator choice in Galali iron mine area, Iran. Int J Agric Crop Sci 5(4):426–432
Niggeweg R, Michael AJ, Martin C (2004) Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat Biotechnol 22(6):746–754
Oláh V, Lakatos G, Bertók C, Kanalas P, Szőllősi E, Kis J, Mészáros I (2010) Short-term chromium (VI) stress induces different photosynthetic responses in two duckwood species, Lemna gibba L. and Lemna minor L. Photosynthetica 48:513–520
Paiva L, Oliveira J, Azevedo R, Ribeiro D, Silva M, Vitoria A (2009) Ecophysiological responses of water hyacinth exposed to Cr3+ And Cr6+. Environ Exp Bot 65:403–409
Pourraut B, Shahid M, Dumat C, Winterton P, Pinelli E (2011) Lead uptake, toxicity and detoxification in plants. Rev Environ Contam T 213:113–136
Prasad MNV (2004) Heavy metal stress in plants. From biomolecules to ecosystems. Springer, Berlin
Prasad DDK, Prasad ARK (1987) Altered delta-aminolevulinic-acid metabolism by lead and mercury in germinating seedlings of bajra (Pennisetum typhoideum). J Plant Physiol 127:241–249
Prasad MNV, Strzalka K (1999) Impact of heavy metals on photosynthesis. In: Prasad MNV, Hagemeyer J (eds) Heavy metal stress in plants. Springer-Verlag, Berlin, pp 117–138
Richardson MD, Hoveland CS, Bacon CW (1993) Photosynthesis and stomatal conductance of symbiotic and nonsymbiotic tall fescue. Crop Sci 33:145–149
Sengar RK, Gautam M, Sengar RK, Grag SK, Sengar K, Chaudhary R (2008) Lead stress effects on physiobiochemical activities of higher plants. Rev Environ Contam T 196:73–93
Singh MV (2009) Micronutrient nutritional problems in soils of India and improvement for human and animal health. Ind J Fertil 5:11–16
Stiborova M, Doubravova M, Brezinova A, Friedrich A (1986) Effect of heavy metal ions on growth and biochemical characteristics of photosynthesis of barley. Photosynthetica 20:418–425
Taiz RL, Zeiger E (2006) Plant Physiology, 4th edn. Sinauer Associates, Inc., Sunderland, MA
Taiz L, Zeiger E (2010) Plant Physiology, 5th edn. Sinauer Associates, Inc., Sunderland, MA
Tewari RK, Kumar P, Sharma PN, Bisht SS (2002) Modulation of oxidative stress responsive enzymes by excess cobalt. Plant Sci 162:381–388
Tholen D, Pons T, Voesenek L, Poorter H (2007) Ethylene insensitivity results in down-regulation of Rubisco expression and photosynthetic capacity in tobacco. Plant Physiol 144:1305–1315
Vaillant N, Monnet F, Hitmi A, Sallanon H, Coudret A (2005) Comparative study of responses in four Datura species to a zinc stress. Chemosphere 59:1005–1013
Van Assche FV, Clijsters H (1986) Inhibition of photosynthesis by treatment of Phaseolus vulgaris with toxic concentration of zinc: effects on electron transport and photophosphorylation. Physiol Plantar 66:717–721
Van Assche F, Ceulemans R, Clijsters H (1980) Zinc mediated effects on leaf CO2 diffusion conductances and net photosynthesis in Phaseolus vulgaris L. Photosynth Res 1:171–180
Vernay P, Gauthier-Moussard C, Hitmi A (2007) Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 68:1563–1575
Weich RM, Webb MJ, Loneragan JF (1982) Zinc in membrane function and its role in phosphorus toxicity. In: Scaife A (ed) Proceedings of the ninth plant nutrition coloquium, Warwick. CAB International, Wallingford, pp 710–715
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Wingler A, Purdy S, MacLean A, Pourtau N (2006) The role of sugars in integrating environmental signals during the regulation of leaf senescence. J Exp Bot 57:391–399
Yanqun Z, Yuan L, Jianjun C, Haiyan C, Li Q, Schvartz C (2005) Hyperaccumulation of Pb, Zn and Cd in herbaceous grown on lead–zinc mining area in Yunnan, China. Environ Int 31:755–762
Zarcinas BA, Pongsakul P, McLaughlin MJ, Cozens G (2004) Heavy metals in soils and crops in Southeast Asia 2, Thailand. Environ Geochem Health 26:359–371
Zengin FK, Munzuroglu O (2005) Effect of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol Cracov Bot 47(2):157–164
Acknowledgments
This work was supported by the National Centre for Research and Development under Grant N R12 0065 10.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Feller.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Borowiak, K., Gąsecka, M., Mleczek, M. et al. Photosynthetic activity in relation to chlorophylls, carbohydrates, phenolics and growth of a hybrid Salix purpurea × triandra × viminalis 2 at various Zn concentrations. Acta Physiol Plant 37, 155 (2015). https://doi.org/10.1007/s11738-015-1904-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1904-x