Abstract
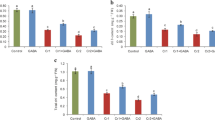
The effect of 100 μM cobalt (Co) on plant growth and on biochemical parameters indicative of oxidative stress was investigated in a hydroponic experiment. The responses of antioxidant enzymes and compounds of the ascorbate–glutathione (AsA–GSH) cycle were also assessed on the hyperaccumulating plant, Indian mustard (Brasssica juncea L.). The effect of excess Co was associated with a significant increase in the levels of proline, carbonylated protein, malondialdehyde, superoxide anion (O ·−2 ), and hydrogen peroxide (H2O2), and resulted in the accumulation of Co. Co toxicity was associated with an increase in the volume of palisade and spongy cells, and a reduction in the number of chloroplasts per cell. Co-induced cell death was characterized by DNA fragmentation and a 36 kDa DNase activity. Despite decreased catalase activity, peroxidase, superoxide dismutase, and AsA–GSH cycle-related enzymes including monodehydroascorbate reductase, dehydroascorbate reductase, and glutathione reductase exhibited remarkable induction under Co stress. Furthermore, the contents of reduced and oxidized forms of ascorbate and glutathione were significantly increased with Co supplementation. Co treatment led to the activation of 44 and 46 kDa mitogen-activated protein kinase (MAPK) and indicated the role of the MAPK cascade in transducing Co-mediated signals. The present results suggest that excess Co reduces seedling growth by inducing oxidative stress related to lipid peroxidation and overproduction of O ·−2 and H2O2. The stimulated activities of antioxidative enzymes and induction of MAPKs did not reverse the oxidative stress caused by Co-induced reactive oxygen species generation in Indian mustard seedlings.




Similar content being viewed by others
Abbreviations
- AA:
-
Reduced ascorbate
- AsA–GSH:
-
Ascorbate–glutathione cycle
- CAT:
-
Catalase
- DHA:
-
Oxidized ascorbate
- DHAR:
-
Dehydroascorbate reductase
- GR:
-
Glutathione reductase
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- LP:
-
Lipid peroxidation
- MAPK:
-
Mitogen-activated protein kinase
- MBP:
-
Myelin basic protein
- MDA:
-
Malondialdehyde
- MDHAR:
-
Monodehydroascorbate reductase
- O ·−2 :
-
Superoxide anion
- OH·:
-
Hydroxyl radical
- PAGE:
-
Polyacrylamide gel electrophoresis
- PCD:
-
Programmed cell death
- POX:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Able AJ, Guest DJ, Sutherland MW (1998) Use of a new tetrazolium-based assay to study the production of superoxide radicals by tobacco cell cultures challenged with avirulent zoospores of Phytophthora parasitica var nicotianae. Plant Physiol 117:491–499
Anderson ME (1985) Determination of glutathione and glutathione disulphide in biological samples. Met Enzymol 113:548–555
Arnon DI (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Arrigoni O (1994) Ascorbate system in plant development. J Bioenerg Biomembr 26:407–419
Asada K (1994) Production and action of active oxygen species in photosynthetic tissue. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and melioration of defense systems in plants. CRC Press, Boca Raton, pp 77–104
Baryla A, Carrier P, Franck F, Coulomb C, Sahut C, Havaux M (2001) Leaf chlorosis in oilseed rape plants (Brassica napus) grown on cadmium-polluted soil: causes and consequences for photosynthesis and growth. Planta 212:696–709
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Behboodi BS, Samadi L (2004) Detection of apoptotic bodies and oligonucleosomal DNA fragments in cadmium-treated root apical cells of Allium cepa Linnaeus. Plant Sci 167:411–416
Boscolo P, Menossi M, Jorge RA (2003) Aluminum-induced oxidative stress in maize. Phytochemistry 62:181–189
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Cavalcanti FR, Lima JP, Ferreira-Silva SL, Viégas RA, Silveira JA (2007) Roots and leaves display contrasting oxidative response during salt stress and recovery in cowpea. J Plant Physiol 164:591–600
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Met Enzymol 11:764–775
Chatterjee J, Chatterjee C (2000) Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ Pollut 109:69–74
Chatterjee J, Chatterjee C (2003) Management of phytotoxicity of cobalt in tomato by chemical measures. Plant Sci 164:793–801
de Gara L, Paciolla C, Tommasi F, Liso R, Arrigoni O (1994) In inhibition of galactono-c-lactone conversion to ascorbate by lycorine. J Plant Physiol 144:649–653
de Vries MPC, Tiller KG (1980) Routine procedures for determining Cu, Zn, Mn and Fe in plant materials, Division of soils divisional report, No. 50. Division of Soils, Commonwealth Scientific and Industrial Research Organization, Australia
Deckert J (2008) Modulation of gene expression in plants exposed to heavy metals. In: Khan NA, Singh S (eds) Abiotic stress and plant responses. I.K. International, pp 125–138
Desnues B, Cuny C, Gregori G, Dukan S, Aguilaniu H, Nystrom T (2003) Differential oxidative damage and expression of stress defence regulons in culturable and non-culturable Escherichia coli cells. EMBO Rep 4:400–404
Diaz J, Barnal A, Pomar F, Merino F (2001) Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci 161:179–188
Doulis AG, Debian N, Kingston-Smith AH, Foyer CH (1997) Differential localization of antioxidants in maize leaves. Plant Physiol 114:1031–1037
Garnier L, Simon-Plas F, Thuleau P, Agnel JP, Blein JP, Ranjeva R, Montillet JL (2006) Cd affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ 29:1956–1969
Gopal R, Dube BK, Sinha P, Chatterjee C (2003) Cobalt toxicity effects on growth and metabolism of tomato. Commun Soil Sci Plant Anal 34:619–628
Heath RL, Packer L (1968) Photoperoxidation of isolated chloroplasts. I. Kinetics and stoichiometry of fatty acids peroxidation. Arch Biochem Biophys 125:189–198
Hoagland DR, Arnon DI (1950) The water-culture for growing plants without soil. Calif Agric Exp Stn Circ 347:1–32
Huang T, Huang H (2008) ROS and CDPK-like kinase-mediated activation of MAP kinase in rice roots exposed to lead. Chemosphere 71:1377–1385
Hung W-C, Huang D-D, Chien P-S, Yeh C-M, Chen P-Y, Chi W-C, Huang H-J (2007) Protein tyrosine dephosphorylation during copper-induced cell death in rice roots. Chemosphere 69:55–62
Hussain MA, Nakano Y, Asada K (1984) Monodehydroacorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Jonak C, Nakagami H, Hirt H (2004) Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol 136:3276–3283
Kaplan J-C, Beutler E (1967) Electrophoresis of red cell NADH and NADPH-diaphorases in normal subjects and patients with congenital methemoglobinemia. Biochem Biophys Res Commun 29:605–610
Karuppanapandian T, Manoharan K (2008) Uptake and translocation of tri- and hexa-valent chromium and their effects on black gram (Vigna mungo L. Hepper cv. Co4) roots. J Plant Biol 51:192–201
Karuppanapandian T, Sinha PB, Kamarul Haniya A, Manoharan K (2006) Differential antioxidative responses of ascorbate–glutathione cycle enzymes and metabolites to chromium stress in green gram (Vigna radiata L. Wilczek) leaves. J Plant Biol 49:440–447
Karuppanapandian T, Sinha PB, Kamarul Haniya A, Manoharan K (2009) Chromium-induced accumulation of peroxide content, stimulation of antioxidative enzymes and lipid peroxidation in green gram (Vigna radiata L. cv. Wilczek) leaves. Afr J Biotechnol 8:475–479
Karuppanapandian T, Moon J-C, Kim C, Manoharan K, Kim W (2011a) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci 5:709–725
Karuppanapandian T, Wang HW, Prabakaran N, Jeyalakshmi K, Kwon M, Manoharan K, Kim W (2011b) 2,4-Dichlorophenoxyacetic acid-induced leaf senescence in mung bean (Vigna radiata L. Wilczek) and senescence inhibition by co-treatment with silver nanoparticles. Plant Physiol Biochem 49:168–177
Kim J-A, Agrawal GK, Rakwal R, Han K-S, Kim K-N, Yun CH, Heu S, Park S-Y, Lee Y-H, Jwa N-S (2003) Molecular cloning and mRNA expression analysis of a novel rice (Oryza sativa L.) MAPK kinase kinase, OsEDR1, an ortholog of Arabidopsis AtEDR1, reveal its role in defense/stress signaling pathways and development. Biochem Biophys Res Commun 300:868–876
Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plats. Proc Natl Acad Sci USA 97:2940–2945
Lanaras T, Moustakas M, Symeonidis L, Diamatoglou S, Karataglis S (1993) Plant metal content, growth responses and some photosynthetic measurements on field cultivated wheat growing on ore bodies enriched in Cu. Physiol Plant 88:307–314
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A, Ahn B, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 106:464–478
Li H-F, Gray C, Mico C, Zhao F-J, McGrath SP (2009) Phytotoxicity and bioavailability of cobalt to plants in a range of soils. Chemosphere 75:979–986
Link V, Hofmann MG, Sinha AK, Ehness R, Strnad M, Roitsch T (2002) Biochemical evidence for the activation of distinct subsets of mitogen-activated protein kinases by voltage and defense-related stimuli. Plant Physiol 128:271–281
Liu J, Reid RJ, Smith FA (2000) The mechanism of cobalt toxicity in mung beans. Physiol Plant 110:104–110
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maksymiec W (2007) Signalling responses in plants to heavy metal stress. Acta Physiol Plant 29:177–187
Maksymiec W, Drazkiewicz M, Skorzynska-Polit E (2008) Responses of higher plants to heavy metal. In: Khan NA, Singh S (eds) Abiotic stress and plant responses. I.K. International, pp 139–163
Manoharan K, Karuppanapandian T, Sinha PB, Prasad R (2005) Membrane degradation, accumulation of phosphatidic acid, stimulation of catalase activity and nuclear DNA fragmentation during 2,4-D-induced leaf senescence in mustard. J Plant Biol 48:394–403
Mansour MMF (2000) Nitrogen containing compounds and adaptation of plants to salinity stress. Biol Plant 43:491–500
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Mei L, Qin Z, Chang-wei H, Li C, Zhi-li L, Zhi-ming K (2007) Cobalt and manganese stress in the microalga Pavlova viridis (Prymnesiophyceae): effects on lipid peroxidation and antioxidant enzymes. J Environ Sci 19:1330–1335
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Mittler R, Vanderauwera S, Gollery M, Van Breusegam F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Neill SJ, Desikan D, Clarke A, Hancock JT (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128:13–16
Noctor G, Foyer CH (1998) A re-evaluation of the ATP:NADPH budget during C3 photosynthesis. A contribution from nitrate assimilation and its associated respiratory activity? J Exp Bot 49:1895–1908
Pandey N, Sharma CP (2002) Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci 163:753–758
Pandey N, Pathak GC, Pandey DK, Pandey R (2009) Heavy metals, Co., Ni, Cu, Zn and Cd, produced oxidative damage and evoke differential antioxidative responses in spinach. Braz J Plant Physiol 21:103–111
Paradiso A, Berardino R, de Pinto M, di Toppi LS, Storelli FT, de Gara L (2008) Increase in ascorbate-glutathione metabolism as local and precocious systemic responses induced by cadmium in durum wheat plants. Plant Cell Physiol 49:362–374
Patterson BD, MacRae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139:487–492
Pena LB, Pasquini LA, Tomaro ML, Gallego SM (2006) Proteolytic system in sunflower (Helianthus annuus L.) leaves under cadmium stress. Plant Sci 171:531–537
Schaedle M, Bassham J (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Schat H, Sharma SS, Vooijs R (1997) Heavy metal-induced accumulation of free proline in metal-tolerant and a nontolerant ecotype of Silene vulgaris. Physiol Plant 101:477–482
Seevers PM, Daly JM, Catedral FF (1971) The role of peroxidase isozymes in resistance to wheat stem rust disease. Plant Physiol 48:353–360
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26:2027–2038
Signorelli S, Arellano JB, Melo TB, Borsani O, Monza J (2013) Proline does not quench singlet oxygen: evidence to reconsider its protective role in plants. Plant Physiol Biochem 64:80–83
Singh R, Tripathi RD, Dwivedi S, Kumar A, Trivedi PK, Chakrabarty D (2010) Lead bioaccumulation potential of an aquatic macrophyte Najas indica are related to antioxidant system. Bioresour Technol 101:3025–3032
Tena G, Asai T, Chiu WL, Sheen J (2001) Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol 4:392–400
Tewari RK, Kumar P, Sharma PN, Bisht SS (2002) Modulation of oxidative stress responsive enzymes by excess cobalt. Plant Sci 162:381–388
Vajpayee P, Rai UN, Ali MB, Tripati RD, Yadav V, Sinha S, Singh SN (2001) Chromium-induced physiologic changes in Vallisneris spiralis L. and its role in phytoremediation of tannery effluents. Bull Environ Contam Toxicol 67:246–256
Woodbury W, Spencer AK, Stahmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 44:301–305
Yeh CM, Hung WC, Huang HJ (2003) Copper treatment activates mitogen-activated protein kinase signalling in rice. Physiol Plant 119:392–399
Zhang S, Liu Y (2001) Activation of salicylic acid-induced protein kinase, a mitogen-activated protein kinase induces multiple defense responses in tobacco. Plant Cell 13:1877–1889
Acknowledgments
Mrs. Sathiya Karuppanapandian is gratefully acknowledged for assistance in conducting the experiments. This work was financially supported by a Korea University Grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Z. Miszalski.
Rights and permissions
About this article
Cite this article
Karuppanapandian, T., Kim, W. Cobalt-induced oxidative stress causes growth inhibition associated with enhanced lipid peroxidation and activates antioxidant responses in Indian mustard (Brassica juncea L.) leaves. Acta Physiol Plant 35, 2429–2443 (2013). https://doi.org/10.1007/s11738-013-1277-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1277-y