Abstract
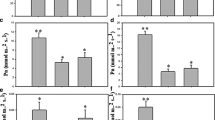
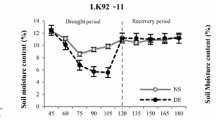
The identification of morpho-physiological traits related to drought tolerance and high yield potential is a challenge when selecting sugar beet genotypes with greater tolerance to water stress. In this paper, root morphological parameters, antioxidant systems, leaf relative water content (RWC) and H+-ATPase activity as key morpho-physiological traits involved in drought tolerance/susceptibility of sugar beet were studied. Genotypes showing a different drought tolerance index (DTI) but a similar yield potential, under moderate (−0.6 Mpa) and severe (−1.2 MPa) water stress, were selected and their morpho-physiological traits were investigated. The results showed a wide genetic variation in morpho-physiological parameters which demonstrated the different adaptive strategies under moderate and severe drought conditions in sugar beet. In particular, an efficient antioxidant system and redox signalling made some sugar beet genotypes more tolerant to drought stress. The alternative strategy of other genotypes was the reduction of root tissue density, which produced a less dense root system improving the axial hydraulic conductivity. These results could be considered as interesting challenge for a better understanding of the drought tolerance mechanisms in sugar beet.







Similar content being viewed by others
References
Ahmadi M, Majidi Heravan E, Sadeghian SY, Mesbah M, Darvish MF (2011) Drought tolerance variability in S1 pollinator lines developed from a sugar beet open population. Euphytica 178:339–349
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Bagatta M, Pacifico D, Mandolino G (2008) Evaluation of the osmotic adjustment response within the genus Beta. J Sugar Beet Res 45:119–133
Barr HD, Weatherley PE (1962) A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust J Biol Sci 15:413–428
Bloch D, Hoffmann C (2005) Seasonal development of genotypic differences in sugar beet (Beta vulgaris L.) and their interaction with water supply. J Agron Crop Sci 191:263–272
Bloch D, Hoffmann CM, Marlander B (2006) Impact of water supply on growth, photosynthesis, water use and carbon isotope discrimination of sugar beet in relation to genotypic variability. Eur J Agron 24:218–225
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen KM, Gong HJ, Wang SM, Zheng WJ, Zhang CL (2005) Regulation of the structure and catalytic properties of plasma membrane H+-ATPase involved in adaptation of two reed ecotypes to their different habitats. Biol Plant 49:513–519
Chołuj D, Karwowska R, Ciszewska A, Jasińska M (2008) Influence of long-term drought stress on osmolyte accumulation in sugar beet (Beta vulgaris L.) plants. Acta Physiol Plant 30(5):679–687
Ciamporova M, Dekankova K, Ovecka M (1998) Intra- and interspecific variation in root length, root turnover and the underlying parameters. In: Lambers H, Poorter H, VanVuuren MMI (eds) Variation in plant growth. Physiological mechanisms and ecological consequences. Backhuys Publishers, Leiden, pp 57–69
Clarke NA, Hetschkun H, Jones C, Boswell E, Marfaing H (1993) Identification of stress tolerance traits in sugar beet. In: Jackson MB, Black CR (eds) Interaction stresses on Plants in a Changing Climate. Springer, Berlin, pp 511–524
Cruz RT, Jordan WR, Drew MC (1992) Structural changes and associated reduction of hydraulic conductance in roots of Sorghum bicolor L. following exposure to water deficit. Plant Physiol 99:203–212
Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inzè D, Van Breusegen F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Fernandez GCJ (1992) Effective selection criteria for assessing plant stress tolerance. In: Kuo CG (ed) Adaptation of fodd crops to temperature and water stress, Publication Number 93-410, Asian Vegetable Research Development Center, Shanhua, Taiwan, p 257–270
Forbusch B (1983) Assay of the Na+-, K+-ATPase in plasma membrane preparations: increasing the permeability of membrane vesicles using sodium dodecylsulfate buffered with bovine serum albumine. Anal Biochem 128:159–163
Gong DS, Xiong YC, Ma BL, Wang TM, Ge JP, Qin XL, Li PF, Kong HY, Li ZZ, Li FM (2010) Early activation of plasma membrane H+-ATPase and its relation to drought adaptation in two contrasting oat (Avena sativa L.) genotypes. Environ Exp Bot 69(1):1–8
Gzik A (1996) Accumulation of proline and pattern of a-amino acids in sugar beet plants in response to osmotic, water and salt stress. Environ Exp Bot 36:29–38
Hernàndez E, Vilagrosa A, Pausas JG, Bellot J (2010) Morphological traits and water use strategies in seedlings of Mediterranean coexisting species. Plant Ecol 207:233–244
Hill JO, Simpson RJ, Moore AD, Chapman DF (2006) Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 286:7–19
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Calif AES Circular 347:1–32
Hoffman CM, Huijbregts T, Swaaji NV, Jansen R (2009) Impact of different environments in Europe on yield and quality of sugar beet genotypes. Eur J Agron 30:17–26
Huang B, Eissenstat DM (2000) Linking hydraulic conductivity to anatomy in plants that vary in specific root length. J Am Soc Hortic Sci 125:260–264
Hummel I, Vile D, Violle C, Devaux J, Ricci B, Blanchard A, Garnier E, Roumet C (2007) Relating root structure and anatomy to whole-plant functioning in 14 herbaceous Mediterranean species. New Phytol 173(2):313–321
Hund A, Reimer R, Trachsel S, Ruta N, Stamp P (2009) Meta-analysis of QTLs controlling early root growth in maize. In: Proceedings of 7th ISRR Symposium ‘Root Research and Applications’, Vienna, Austria p 49
Jahufer MZZ, Nichols SN, Crush JR, Ouyang L, Dunn A, Ford JL, Care DA, Griffiths AG, Jones CS, Jones CG, Woodfield DR (2008) Genotypic variation for root trait morphology in a white clover mapping population grown. Crop Sci 48(2):487–494
Jones PD, Lister DH, Jaggard KW, Pidgeon JD (2003) Future climate change impact on the productivity of sugar beet (Beta vulgaris L.). Eur Clim Change 58:93–108
Joseph G, Kelsey RG, Thies WG (1998) Hydraulic conductivity in roots of ponderosa pine infected with black-stain (Leptographium wageneri) or annosus (Heterobasidion annosum) root disease. Tree Physiol 18:333–339
Kerr S (2000) Variety interactions with sowing, soils and harvest. Brit Sugar Beet Rev 68:18–22
Larcher W (1995) Physiological plant ecology, 3rd edn. Springer, Berlin
Levitt J (1972) Responses of plants to environmental stresses. Academic Press, New York
Liu HP, Yu BJ, Zhang WH, Liu YL (2005) Effect of osmotic stress on the activity of H+-ATPase and the levels of covalently and noncovalently conjugated polyamines in plasma membrane preparation from wheat seedling roots. Plant Sci 168:1599–1607
Luković J, Maksimović I, Zorić L, Nagl N, Perčić M, Polić D, Putnik-Delić M (2009) Histological characteristics of sugar beet leaves potentially linked to drought tolerance. Ind Crop Prod 30:281–286
Manschadi AM, Cristopher G, Devoil P, Hammer GL (2008) The role of root architectural traits in adaptation of wheat to water-limited environments. Funct Plant Biol 33(9):823–837
Märländer B, Hoffmann C, Koch HJ, Ladewig E, Merkes R, Petersen J, Stockfisch N (2003) Environmental situation and yield performance of the sugar beet crop in Germany: heading for sustainable development. J Agron Crop Sci 189:201–226
Michel BE (1983) Evaluation of the water potential of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiol 72:66–70
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant, Cell Environ 33:566–589
Mohammadian R, Khoyi FR, Rahimian H, Moghaddam M, Ghassemi-Golezani K, Sadeghian SY (2001) The effect of early season drought on stomatal conductance, leaf-air temperature difference and proline accumulation in sugar beet genotypes. J Agric Sci Technol 3:181–193
Noldt G, Bauch J, Koch G, Schmitt U (2001) Fine roots of Carapa guianensis Aubl. and Swietenia macrophylla King: cell structure and adaptation to the dry season in Central Amazonia. J Appl Bot 75:152–158
North GB, Nobel PS (1996) Radial hydraulic conductivity of individual root tissues of Opuntia ficus-indica (L.) Miller as soil moisture varies. Ann Bot 77:133–142
Ober ES (2001) The search for drought tolerance in sugar beet. Brit Sugar Beet Rev 69:40–43
Ober ES, Luterbacher MC (2002) Genotypic variation for drought tolerance in Beta vulgaris. Ann Bot 89:917–924
Ober ES, Rajabi A (2011) Abiotic stress in sugar beet. Sugar Tech. doi:10.1007/s12355-010-0035-3
Ober ES, Sharp RE (2003) Electrophysiological responses of maize roots to low water potentials: relationship to growth and ABA accumulation. J Exp Bot 54:813–824
Ober ES, Sharp RE (2007) Regulation of root growth responses to water deficit. In: Jenks MA, Hasegawa PM, Jain SM (eds) Advances in Molecular Breeding toward Drought and Salt Tolerant Crops. Springer, Dortrecht, pp 33–53
Ober ES, Clark CJA, Le Bloa M, Royal A, Jaggard KW, Pidgeon JD (2004) Assessing the genetic resources to improve drought tolerance in sugar beet: agronomic traits of diverse genotypes under droughted and irrigated conditions. Field Crop Res 90:213–234
Ober ES, Le Bloa M, Clark CJA, Royal A, Jaggard KW, Pidgeon JD (2005) Evaluation of physiological traits as indirect selection criteria for drought tolerance in sugar beet. Field Crop Res 91:231–249
Pandolfini T, Gabrielli R, Camporini C (1992) Nickel toxicity and peroxidase activity in seedlings of Triticum aestivum L. Plant Cell Environ 15:719–725
Pemàn J, Voltas J, Gil-Pelegrin E (2006) Morphological and functional variability in the root system of Quercus ilex L. subject to confinement: consequences for afforestation. Ann Forest Sci 63:425–430
Pidgeon JD, Werker AR, Jaggard KW, Richter GM, Lister DH, Jones PD (2001) Climatic impact on the productivity of sugar beet (Beta vulgaris L.) in Europe 1961–1995. Agr Forest Meteorol 109:27–37
Pidgeon JD, Ober ES, Qi A, Clark CJA, Royal A, Jagard KW (2006) Using multi-environment sugar beet variety trials to screen for drought tolerance. Field Crop Res 95:268–279
Pignocchi C, Foyer CH (2003) Apoplastic ascorbate metabolism and its role in the regulation of cell signaling. Curr Opin Plant Biol 6:379–389
Quartacci MF, Navarri-Izzo F (1992) Water stress and free radical mediated changes in sunflower seedlings. J Plant Physiol 139:621–625
Rajabi A, Griffiths H, Ober ES (2009) Carbon isotope discrimination in sugar beet: stability across environments and potential surrogate measures. Field Crop Res 112:172–181
Ryser P (1998) Intra- and interspecific variation in root length, root turnover and the underlying parameters. In: Lambers H, Poorter H, Van Vuuren MMI (eds) Inherent variation in plant growth. Physiological mechanism and ecological consequences. Backhuys Publishers, Leiden, pp 441–465
Ryser P (2006) The mysterious root length. Plant Soil 286:1–6
Ryser P, Lambers H (1995) Root and leaf attributes accounting for the performance of fast and slow-growing grasses at different nutrient supply. Plant Soil 170:251–265
Sadeghian SY, Yavari N (2004) Effect of water-deficit stress on germination and early seedling growth in sugar beet. J Agron Crop Sci 190:138–144
Sadeghian SY, Fazli H, Mohammadian R, Taleghani DF, Mesbah M (2000) Genetic variation for drought stress in sugar beet. J Sugar Beet Res 37:55–77
Sanità di Toppi L, Marabottini R, Vattone Z, Musetti R, Favalli MA, Sorgonà A, Badiani M (2005) Cell wall immobilization and antioxidant status of Xanthoria parietina thalli exposed to cadmium. Funct Plant Biol 32(7):611–618
Santi S, Locci G, Pinton R, Cesco S, Varanini Z (1995) Plasma membrane H+-ATPase in maize roots induced for NO3 − uptake. Plant Physiol 109:1277–1283
Sayfzadeh S, Rashidi M (2010) Effect of Drought Stress on Antioxidant Enzyme Activities and Root Yield of Sugar Beet (Beta vulgaris). Am Eurasian J Agric Environ Sci 9(3):223–230
Sharp RE, LeNoble ME (2002) ABA, ethylene and the control of shoot and root growth under water stress. J Exp Bot 53:33–37
Shaw B, Thomas TH, Cooke DT (2002) Responses of sugar beet (Beta vulgaris L.) to drought and nutrient deficiency stress. Plant Growth Regul 37:77–83
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Sofo A, Dichio B, Xiloyannis C, Masia A (2005) Antioxidant defences in olive tree during drought stress: changes in activity of some antioxidant enzymes. Funct Plant Biol 32(1):45–53
Tsialtas JT, Maslaris N (2012) Leaf physiological traits and their relation with sugar beet cultivar success in two contrasting environments. Int J Plant Prod 6:15–36
Tucker SS, Craine JM, Nippert JB (2011) Physiological drought tolerance and the structuring of tallgrass prairie assemblages. Ecosphere 2:1–19
Vasellati V, Oesterheld M, Medan D, Loreti J (2001) Effects of flooding and drought on the anatomy of Paspalum dilatatum. Ann Bot 88:355–360
Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu JH, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45:523–539
Wahl S, Ryser P (2000) Root tissue structure is linked to ecological strategies of grasses. New Phytol 148(3):459–471
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, Song C-P (2001) Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. V. Jorrin-Novo.
Rights and permissions
About this article
Cite this article
Romano, A., Sorgonà, A., Lupini, A. et al. Morpho-physiological responses of sugar beet (Beta vulgaris L.) genotypes to drought stress. Acta Physiol Plant 35, 853–865 (2013). https://doi.org/10.1007/s11738-012-1129-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1129-1