Abstract
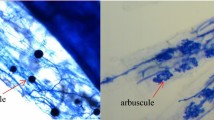
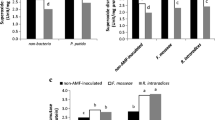
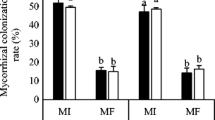
The objective of this study was to investigate the effects of arbuscular mycorrhizal fungus (AMF) inoculation on plant growth and drought tolerance in seedlings of a promising oilseed crop, Sacha Inchi (Plukenetia volubilis L.), under well-watered or drought conditions. AMF inoculation was applied in four treatments: without AMF inoculation, Glomus versiforme, Paraglomus occultum, or combination of both microorganism inoculations. The results showed that AMF colonization significantly enhanced the growth of Sacha Inchi seedlings regardless of soil water conditions, and the greatest development was reached in plants dually inoculated under well-watered conditions. G. versiforme was more efficient than P. occultum. Plants inoculated with both symbionts had significantly greater specific leaf area, leaf area ratio and root volume when compared with the uninoculated control, G. versiforme, and P. occultum treatments alone, indicating a synergistic effect in the two AMF inoculation. Photosynthetic rate and water-use efficiency were stimulated by AMF, but not stomatal conductance. Inoculation with AM fungus increased antioxidant enzymes activities including guaiacol peroxidase and catalase, thus lowering hydrogen peroxide accumulation and oxidative damage, especially under drought stress conditions. However, proline content showed little change during drought stress and AMF colonization conditions, which suggested that proline accumulation might not serve as the main compound for osmotic adjustment of the studied species. These results indicate that AMF inoculation stimulated growth and enhanced drought tolerance of Sacha Inchi seedlings, through alterations in morphological, physiological and biochemical traits. This microbial symbiosis might be an effective cultivation practice in improving the performance and development for Sacha Inchi plants.




Similar content being viewed by others
Abbreviations
- AMF:
-
Arbuscular mycorrhizal fungi
- CAT:
-
Catalase (μmol H2O2 min−1 mg−1)
- Chl:
-
Leaf chlorophyll content (g m−2)
- GPX:
-
Guaiacol peroxidase (μmol min−1 mg−1)
- G s :
-
Stomatal conductance (mol m−2 s−1)
- H2O2 :
-
Hydrogen peroxide (μmol g−1)
- MDA:
-
Malondialdehyde (nmol g−1)
- P max :
-
Light-saturated photosynthetic rate (μmol m−2 s−1)
- RGR:
-
Relative growth rate (mg g−1 day−1)
- ROS:
-
Reactive oxygen species
- SLA:
-
Specific leaf area (cm2 g−1)
- LAR:
-
Leaf area ratio (cm2 g−1)
- WUE:
-
Water-use efficiency (μmol mol−1)
References
Abdel Latef AA, He C (2011) Arbuscular mycorrhizal influence on growth, photosynthetic pigments, osmotic adjustment and oxidative stress in tomato plants subjected to low temperature stress. Acta Physiol Plant 33:1217–1225
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Augé RM (2001) Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42
Babu AG, Reddy MS (2011) Dual inoculation of arbuscular mycorrhizal and phosphate solubilizing fungi contributes in sustainable maintenance of plant health in fly ash ponds. Water Air Soil Pollut 219:3–10
Bago B, Pfeffer PE, Shachar-Hill Y (2000) Carbon metabolism and transport in arbuscular mycorrhizas. Plant Physiol 124:949–958
Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1778
Bates LS, Waldren SP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beligni MV, Lamattina L (1999) Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 208:337–344
Bressano M, Curetti M, Giacheroa L, Gil SV, Cabello M, March G, Ducasse DA, Luna CM (2010) Mycorrhizal fungi symbiosis as a strategy against oxidative stress in soybean plants. J Plant Physiol 167:1622–1626
Cai ZQ (2011) Shade delayed flowering and decreased photosynthesis, growth and yield of Sacha Inchi (Plukenetia volubilis) plants. Ind Crops Prod 34:1235–1237
Cai ZQ, Wang WH, Yang J, Cai CT (2009) Growth, photosynthesis and root reserpine concentrations of two Rauvolfia species in response to a light gradient. Ind Crop Prod 30:220–226
Cai ZQ, Yang Q, Tang SX, Dao XS (2011) Nutritional evaluation in seeds of a woody oil crop, Plukenetia volubilis Linneo. Acta Nutr Sin 33:193–195
Cai ZQ, Jiao DY, Tang SX, Dao XS, Lei YB, Cai CT (2012) Leaf photosynthesis, growth and seed chemicals of Sacha Inchi (Plukenetia volubilis) plants cultivated along an altitude gradient. Crop Sci 52:1859–1867
Chaves M, Maroco JP, Pereira JS (2003) Understanding plant responses to drought—from genes to the whole plant. Funct Plant Biol 30:239–264
Cruz C, Green JJ, Watson CA, Wilson F, Martins-Loucao MM (2004) Functional aspects of root architecture and mycorrhizal inoculation with respect to nutrient uptake capacity. Mycorrhiza 14:177–184
Garcia AB, Engler JDA, Iyer S, Gerats T, Van Montagu M, Caplan AB (1997) Effects of osmoprotectants upon NaCl stress in rice. Plant Physiol 115:159–169
Hamaker BRC, Valles R, Gilman RM et al (1992) Amino acid and fatty acid profiles of the Inca peanut (Plukenetia volubilis L.). Cereal Chem 69:461–463
Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21:535–553
Hodges DM, Andrews CJ, Johnson DA, Hamilton RI (1999) Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiol Plant 98:685–692
Huang Z, Zou ZR, He CX, He ZQ, Zhang ZB, Li JM (2011) Physiological and photosynthetic responses of melon (Cucumis melo L.) seedlings to three Glomus species under water deficit. Plant Soil 339:391–399
Lei YB, Yin CY, Li CY (2006) Differences in some morphological, physiological, and biochemical responses to drought stress in two contrasting populations of Populus przewalskii. Physiol Plant 127:182–191
Lei YB, Feng YL, Zheng YL, Wang RF, Gong HD, Zhang YP (2011) Innate and evolutionary advantages of invasive Eupatorium adenophorum over native E. japonicum under ambient and doubled atmospheric CO2 concentrations. Biol Invasions 13:2703–2714
Lin J, Wang G (2002) Doubled CO2 could improve the drought tolerance better in sensitive cultivars than in tolerant cultivars in spring wheat. Plant Sci 63:627–637
Lutts S, Majerus V, Kinet JM (1999) NaCl effects on proline metabolism in rice (Oryza sativa) seedlings. Physiol Plant 105:450–458
Navarro A, Sanchez-Blanco MJ, Mortec A, Banon S (2009) The influence of mycorrhizal inoculation and paclobutrazol on water and nutritional status of Arbutus unedo L. Environ Exp Bot 66:362–371
Pagano MC, Scotti MR, Cabello MN (2009) Effect of the inoculation and distribution of mycorrhizae in Plathymenia reticulata Benth under monoculture and mixed plantation in Brazil. New Forest 38:197–214
Poorter L (1999) Growth responses of 15 rain-forest tree species to a light gradient: the relative importance of morphological and physiological traits. Funct Ecol 13:396–410
Poorter H, Miinemets U, Poorter L (2009) Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol 182:565–588
Reis FS, Ferreiral ICFR, Barros L (2011) Mycorrhizal induction of phenolic compounds and antioxidant properties of fungi and seedlings during the early steps of symbiosis. Chemoecology 21:151–159
Ruíz-Sáncheza M, Armadab E, Munoza Y, Salamonec IE, Arocab R, Ruíz-Lozanob JM, Azcónb R (2011) Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J Plant Physiol 168:1031–1037
Schellenbaum L, Berta G, Ravolanirina F, Tisserant B, Gianinazzi S, Fitter AH (1991) Influence of endomycorrhizal infection on root morphology in a micro-propagated woody plant species (Vitis vinifera L.). Ann Bot 68:135–141
Smith SE, Read DJ, Venekamp JH (2008) Mycorrhizal symbiosis, 3rd edn. Academic, London
Smith SE, Jakobsen I, Gronlund M, Simth FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol 156:1050–1057
Wang J, Huang Y, Jiang XY (2011) Influence of ectomycorrhizal fungi on absorption and balance of essential elements of Pinus tabulaeformis seedlings in saline soil. Pedosphere 21:400–406
Wu QS, Xia RX (2006) Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 163:417–425
Zhang Y, Zhong CL, Chen Y, Chen Z, Jiang QB, Wu C, Pinyopusarerk K (2010) Improving drought tolerance of Casuarina equisetifolia seedlings by arbuscular mycorrhizas under glasshouse conditions. New Forest 40:261–271
Acknowledgments
This work was financially supported by grants (KSCX2EWQ17, KSCX2EWZ15) from Chinese Academy of Sciences, and by grants (30900220, 31170641) from the National Science Foundation in China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. H. Walter.
Rights and permissions
About this article
Cite this article
Tian, Yh., Lei, Yb., Zheng, Yl. et al. Synergistic effect of colonization with arbuscular mycorrhizal fungi improves growth and drought tolerance of Plukenetia volubilis seedlings. Acta Physiol Plant 35, 687–696 (2013). https://doi.org/10.1007/s11738-012-1109-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1109-5