Abstract
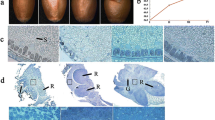
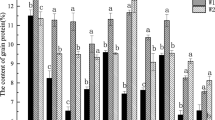
To identify specific proteins related to maize seed viability, seeds of Zhengdan 958 (one of the high-yield maize hybrids in China) were sorted based on viability evaluation with triphenyltetrazolium chloride (TTC) assay and used for comparative proteomic analysis. After TTC staining, embryos of high-viability seeds were deep red (R type), while embryos of dead seeds were white (W type). Proteomic analysis revealed that 28 protein spots identified were differently expressed significantly between R and W embryos, of which 20 were up-regulated and 8 down-regulated in R embryos. Among them were proteins involved in stress response, protein folding, and stabilization, as wells as proteins related to nutrient reservoir and metabolism. Prominently, small heat shock proteins, late embryogenesis abundant (LEA) proteins, and antioxidant enzymes were highly up-regulated, while two proteases were highly down-regulated in R embryos compared to W embryos. One of LEA proteins was EMB564, which declined in abundance during artificial aging of seeds. Our results suggested an association of EMB564 with maize seed viability. It would be of interest to use these small proteins to develop quick tests for seed quality.




Similar content being viewed by others
Abbreviations
- 2-D:
-
Two-dimensional
- 2-DE:
-
Two-dimensional electrophoresis
- CBB:
-
Coomassie brilliant blue
- DTT:
-
Dithiothreitol
- CDT:
-
Controlled deterioration treatment
- GST:
-
Glutathione S-transferase
- IEF:
-
Isoelectric focusing
- LEA:
-
Late embryogenesis abundant
- M:
-
Medium red type
- MALDI-TOF:
-
Matrix-assisted laser desorption/ionization time of flight
- MDA:
-
Malondialdehyde
- Ms:
-
Mass spectrometry
- R:
-
Red type
- sHSPs:
-
Small heat shock proteins
- SOD:
-
Superoxide dismutase
- TCA:
-
Trichloroacetic acid
- TTC:
-
Triphenyltetrazolium chloride
- W:
-
White type
References
Alexandrov NN, Brover VV, Freidin S, Troukhan ME, Tatarinova TV, Zhang HY et al (2009) Insights into corn genes derived from large-scale cDNA sequencing. Plant Mol Biol 69:179–194
Battaglia M, Olvera-carrillo Y, Garciarrubio A, Campos F, Covarrubias AA (2008) The enigmatic LEA proteins and other hydrophilins. Plant Physiol 148:6–24
Bettey M, Finch-Savage WE, King GJ, Campos F, Covarrubias AA (2000) Quantitative genetic analysis of seed viability and pre-emergence seedling growth traits in Brassica oleracea. New Phytol 148:277–286
Boudet J, Buitink J, Hoekstra FA, Rogniaux H, Larre C, Satour P et al (2006) Comparative analysis of the heat stable proteome of radicles of Medicago truncatula seeds during germination identifies late embryogenesis abundant proteins associated with desiccation tolerance. Plant Physiol 140:1418–1436
Brini F, Hanin M, Lumbreras V, Amara I, Khoudi H, Hassairi A et al (2007) Overexpression of wheat dehydrin DHN-5 enhances tolerance to salt and osmotic stress in Arabidopsis thaliana. Plant Cell Rep 26:2017–2026
Campo S, Carrascal M, Coca M, Abian J, San Segundo B (2004) The defense response of germinating maize embryos against fungal infection: a proteomics approach. Proteomics 4:383–396
Catusse J, Strub JM, Job C, Van Dorsselaer A, Job D (2008) Proteome-wide characterization of sugar beet seed viability and its tissue specific expression. Proc Natl Acad Sci USA 105:10262–10267
Dure L, Crouch M, Harada JJ, Ho THD, Mundy J, Quatrano R et al (1989) Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol Biol 12:475–486
Espelund M, Saeboe-Larssen S, Hughes DW, Galau GA, Larsen F, Jakobsen KS (1992) Late embryogenesis-abundant genes encoding proteins with different numbers of hydrophilic repeats are regulated differentially by abscisic acid and osmotic stress. Plant J 2:241–252
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Ann Rev Physiol 61:243–282
Fujita Y, Fujita M, Satoh R, Maruyama K, Parvez MM, Seki M et al (2005) AREB1 is a transcription activator of novel ABRE-dependent ABA signaling that enhances drought stress tolerance in Arabidopsis. Plant Cell 17:3470–3488
Gallardo K, Job C, Groot SPC, Puype M, Demol H, Vandekerckhove J et al (2001) Proteomic analysis of Arabidopsis seed germination and priming. Plant Physiol 126:835–848
Galleschi L, Capocchi A, Ghiringhelli S, Saviozzi F (2002) Antioxidants, free radicals, storage proteins, and proteolytic activities in wheat (Triticum durum) seeds during accelerated aging. J Agric Food Chem 50:5450–5457
Heath RL, Parker L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Job CL, Lovigny Y, Belghazi M, Belghazi M, Job D (2005) Patterns of protein oxidation in Arabidopsis seeds and during germination. Plant Physiol 138:790–802
Kalemba EM, Pukacka S (2007) Possible roles of LEA proteins and sHSPs in seed protection: a short review. Biol Lett 44:3–16
Kocsy G, Von Ballmoos P, Ruegsegger A, Szalai G, Galiba G, Brunold C (2001) Increasing the glutathione content in a chilling-sensitive maize genotype using safeners increased protection against chilling-induced injury. Plant Physiol 127:1147–1156
Kumar GNM, Houtz RL, Knowles NR (1999) Age-induced protein modifications and increased proteolysis in potato seed-tubers. Plant Physiol 119:89–99
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105–132
Linding R, Russell RB, Neduva V, Gibson TJ (2003) GlobPlot: exploring protein sequences for globularity and disorder. Nucleic Acids Res 31:3701–3708
Linn SS, Pearce RS (1990) Changes in lipids in bean seeds (Phaseolus vulgaris) and corn caryopses (Zea mays) aged in contrasting environments. Ann Bot 65:452–456
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Manfre AJ, Lanni LM, Marcotte WR (2006) The Arabidopsis group 1 late embryogenesis abundant protein ATEM6 is required for normal seed development. Plant Physiol 140:140–149
McDonald MB (1999) Seed deterioration: physiology, repair and assessment. Seed Sci Technol 27:177–237
McDonough CM, Floyd CD, Waniska RD, Rooney LW (2004) Effect of accelerated aging on maize, sorghum, and sorghum meal. J Cereal Sci 39:351–361
Melonia DA, Oliva MA, Martinez CA, Cambraia J (2003) Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ Exp Bot 49:69–76
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Ogé L, Bourdais G, Bove J, Collet B, Godin B, Granier F et al (2008) Protein repair L-isoaspartyl methyltransferase 1 is involved in both seed longevity and germination viability in Arabidopsis. Plant Cell 20:3022–3037
Pukacka S, Ratajczak E (2007) Age-related biochemical changes during storage of beech (Fagus sylvatica L.) seeds. Seed Sci Res 17:45–53
Rajesh S, Manickam A (2006) Prediction of functions for two LEA proteins from mung bean. Bioinformation 1:133–138
Rajjou L, Belghazi M, Huguet R, Robin C, Moreau A, Job C et al (2006) Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol 141:910–923
Rajjou L, Lovigny Y, Groot SPC, Belghazi M, Job C, Job D (2008) Proteome-wide characterization of seed aging in Arabidopsis: a comparison between artificial and natural aging protocols. Plant Physiol 148:620–641
Ramanjulu S, Bartels D (2002) Drought- and desiccation-induced modulation of gene expression in plants. Plant Cell Environ 25:141–151
Revilla R, Butrón A, Rodríguez VM, Malvar RA, Ordás A (2009) Identification of genes related to germination in aged maize seed by screening natural variability. J Exp Bot 60:4151–4157
Richter AK, Frossard E, Brunner I (2007) Polyphenols in the woody roots of Norway spruce and European beech reduce TTC. Tree Physiol 27:155–160
Shin JH, Kim SR, An G (2009) Rice aldehyde dehydrogenase7 is needed for seed maturation and viability. Plant Physiol 149:905–915
Wang W, Vignani R, Scali M, Sensi E, Tiberi P, Cresti M (2006) A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 27:2782–2786
Wang JH, Chen SN, Bai B, Tang JH, Wang W (2009a) Effects of artificial aging on seed viability and proteome of maize seeds. J Henan Agric Univ 43:132–135
Wang W, Li CQ, Hu XL (2009b) Developmental expression of β-glucosidase in olive leaves. Biol Plant 53:138–140
Wang W, Bianchi L, Scali M, Liu LW, Bini L, Cresti M (2009c) Proteomic analysis of β-1, 3-glucanase in grape berry tissues. Acta Physiol Plant 31:597–604
Wehmeyer N, Vierling E (2000) The expression of small heat shock proteins in seeds responds to discrete development signals and suggests a general protective role in desiccation tolerance. Plant Physiol 122:1099–1108
Williams BA, Tsang A (1991) A maize gene expressed during embryogenesis is abscisic acid-inducible and highly conserved. Plant Mol Biol 16:919–923
Williams BA, Tsang A (1994) Analysis of multiple classes of abscisic acid responsive genes during embryogenesis in Zea mays. Dev Genet 15:415–424
Zhao PF, Zhu YH, Wang W (2010) Evaluation and improvement of spectrophotometric assays of TTC reduction: maize (Zea mays) embryo as an example. Acta Physiol Plant. doi:10.1007/s11738-009-0457-2 (in press)
Zolla L, Rinalducci S, Antonioli P, Righetti PG (2008) Proteomics as a complementary tool for identifying unintended side effects occurring in transgenic maize seeds as a result of genetic modifications. J Proteome Res 7:1850–1861
Zur Nieden U, Neumann D, Bucka A, Neumann D (1995) Tissue-specific localization of heat-stress proteins during embryo development. Planta 196:530–538
Acknowledgments
This work was supported by the Program for Science and Technology Innovation Talents in Universities of Henan Province (project 2008HASTIT005), by Innovation Scientists and Technicians Troop Construction Projects of Henan Province (project 9410051003), and partly by the National Natural Science Foundation of China (project 30971705).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Z.-L. Zhang.
Rights and permissions
About this article
Cite this article
Wu, X., Liu, H., Wang, W. et al. Proteomic analysis of seed viability in maize. Acta Physiol Plant 33, 181–191 (2011). https://doi.org/10.1007/s11738-010-0536-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0536-4