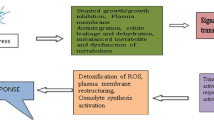
Abstract
Low temperature is one of the major abiotic stresses limiting the productivity and the geographical distribution of many important crops. Many plants increase in freezing tolerance in response to low temperatures. This phenomenon needs a vast reprogramming of gene expression which results in the adjusted metabolic-structural alterations. However, the efficient adjustments are dependent on proper cold signal transduction. The first stage is cold stress signal perception which is carried out by different pathways. Transcriptional cascades are next players which operate through ABA-dependent and ABA-independent pathways to induce cold-regulated (COR) gene expression and the result is increasing in the levels of hundreds of metabolites, which some of them are known to have protective effects against the damaging effects of cold stress and some like soluble sugars, reactive oxygen species and photosynthetic metabolites are thought to act as signaling molecules and regulate special COR genes. The different aspects of these events are discussed in detail below.
Similar content being viewed by others
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Atici O, Nalbantoglu B (2003) Antifreeze proteins in higher plants. Phytochemistry 64:1187–1196
Badawi M, Reddy YV, Agharbaoui Z, Tominaga Y, Danyluk J, Sarhan F, Houde M (2008) Structure and functional analysis of wheat ICE (inducer of CBF expression) genes. Plant Cell Physiol 49:1237–1249
Beck EH, Heim R, Hansen J (2004) Plant resistance to cold stress: mechanisms and environmental signals triggering frost hardening and dehardening. J Biosci 29:449–459
Beck EH, Fettig S, Knake C, Hartig K, Bhattarai T (2007) Specific and unspecific responses of plants to cold and drought stress. J Biosci 32:501–510
Carpaneto A, Ivashikina N, Levchenko V, Krol E, Jeworutzki E, Zhu JK, Hedrich R (2007) Cold transiently activates calcium-permeable channels in Arabidopsis mesophyll cells. Plant Physiol 143:487–494
Charron JBF, Ouellet F, Pelletier M, Danyluk J, Chauve C, Sarhan F (2005) Identification, expression, and evolutionary analyses of plant lipocalins. Plant Physiol 139:2017–2028
Chen M, Wang QY, Cheng XG, Xu ZS, Li LC, Ye XG, Xia LQ, Ma YZ (2007) GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem Biophys Res Commun 353:299–305
Chen M, Xu Z, Xia L, Li L, Cheng X, Dong J, Wang Q, Ma Y (2009) Cold-induced modulation and functional analyses of the DRE-binding transcription factor gene, GmDREB3, in soybean (Glycine max L.). J Exp Bot 60:121–135
Chinnusamy V, Zhu JK (2002) Molecular genetic analysis of cold-regulated gene transcription. Phil Trans R Soc Lond B 357:877–886
Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK (2003) ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 17:1043–1054
Chinnusamy V, Schumaker K, Zhu JK (2004) Molecular genetic perspectives on cross-talk and specifcity in abiotic stress signalling in plants. J Exp Bot 55:225–236
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12:444–451
Choi HI, Hong JH, Ha JO, Kang JY, Kim SY (2000) ABFs, a family of ABA-responsive element binding factors. J Biol Chem 275:1723–1730
Close TJ (1996) Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiol Plant 97:795–803. doi:10.1111/j.1399-3054.1996.tb00546.x
Colcombet J, Hirt H (2008) Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. Biochem J 413:217–226
Danyluk J, Perron A, Houde M, Limin A, Fowler B, Benhamou N, Sarhan F (1998) Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during cold acclimation of wheat. Plant Cell 10:623–638
Dat J, Vandenabeele S, Vranova E, Van Montagu M, Inzé D, Van Breusegem F (2000) Dual action of the active oxygen species during plant stress responses. Cell Mol Life Sci 57:779–795
Davletova S, Schlauch K, Coutu J, Mittler R (2005) The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol 139:847–856
Deryabin AN, Dubinina IM, Burakhanova EA, Astakhova NV, Sabel’nikova EP, Trunova TI (2005) Influence of yeast-derived invertase gene expression in potato plants on membrane lipid peroxidation at low temperature. J Therm Biol 30:73–77. doi:10.1016/j.jtherbio.2004.07.002
Desikan R, Mackerness SAH, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127:159–172
Dhonukshe P, Laxalt AM, Goedhart J, Gadella TWJ, Munnik T (2003) Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell 15:2666–2679
Ding JP, Pickard BG (1993) Modulation of mechanosensitive calcium-selective cation channels by temperature. Plant J 3:713–720
Dodd AN, Jakobsen MK, Baker AJ, Telzerow A, Hou SW, Laplaze L, Barrot L, Poethig RS, Haseloff J, Webb AAR (2006) Time of day modulates low-temperature Ca2+ signals in Arabidopsis. Plant J 48:962–973
Drøbak BK, Franklin-Tong VE, Staiger CJ (2004) The role of the actin cytoskeleton in plant cell signaling. New Phytol 163:13–30. doi:10.1111/j.1469-8137.2004.01076.x
Ensminger I, Busch F, Huner NPA (2006) Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiol Plant 126:28–44. doi:10.1111/j.1399-3054.2005.00627.x
Ganeshan S, Vitamvas P, Fowler DB, Chibbar RN (2008) Quantitative expression analysis of selected COR genes reveals their differential expression in leaf and crown tissues of wheat (Triticum aestivum L.) during an extended low temperature acclimation regimen. J Exp Bot 59:2393–2402
Gardiner JC, Harper JDI, Weerakoon ND, Collings DA, Ritchie S, Gilroy S, Cyr RJ, Marc J (2001) A 90-kD phospholipase D from tobacco binds to microtubules and the plasma membrane. Plant Cell 13:2143–2158
Gardiner J, Collings DA, Harper JDI, Marc J (2003) The effects of the phospholipase D-antagonist 1-butanol on seedling development and microtubule organisation in Arabidopsis. Plant Cell Physiol 44:687–696
George L, Romanowsky SM, Harper JF, Sharrock RA (2008) The ACA10 Ca2+-ATPase regulates adult vegetative development and inflorescence architecture in Arabidopsis. Plant Physiol 146:716–728
Griffith M, Antikainen M, Hon WC, Pihakaski-Maunsbach K, Yu XM, Chun JU, Yang DSC (1997) Antifreeze proteins in winter rye. Physiol Plant 100:327–332. doi:10.1111/j.1399-3054.1997.tb04790.x
Guy C (1999) Molecular responses of plants to cold shock and cold acclimation. J Mol Microbiol Biotechnol 1:231–242
Guy C, Kaplan F, Kopka J, Selbig J, Hincha DK (2008) Metabolomics of temperature stress. Physiol Plant 132:220–235
Hara M, Fujinaga M, Kuboi T (2005) Metal binding by Citrus dehydrin with histidine-rich domains. J Exp Bot 56:2695–2703
Heath RJ, Rock CO (1996) Regulation of fatty acid elongation and initiation by acyl-acyl carrier protein in Escherichia coli. J Biol Chem 271:1833–1836
Hon WC, Griffith M, Mlynarz A, Kwok YC, Yang DSC (1995) Antifreeze proteins in winter rye are similar to pathogenesis-related proteins. Plant Physiol 109:879–889
Hong Y, Zheng S, Wang X (2008) Dual functions of phospholipase Dα1 in plant response to drought. Mol Plant 1:262–269. doi:10.1093/mp/ssm025
Houde M, Belcaid M, Ouellet F, Danyluk J, Monroy AF, Dryanova A, Gulick P, Bergeron A, Laroche A, Links MG, MacCarthy L, Crosby WL, Sarhan F (2006) Wheat EST resources for functional genomics of abiotic stress. BMC Genomics 7:149. doi:10.1186/1471-2164-7-149
Humphrey TV, Bonetta DT, Goring DR (2007) Sentinels at the wall: cell wall receptors and sensors. New Phytol 176:7–21
Hung SH, Yu CW, Lin CH (2005) Hydrogen peroxide functions as a stress signal in plants. Bot Stud 46:1–10
Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K (2000) Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMK4 and ATMK6. Plant J 24:655–665
Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, Kreis M, Zhang S, Hirt H, Wilson C, Heberle-Bors E, Ellis BE, Morris PC, Innes RW, Ecker JR, Scheel D, Klessig DF, Machida Y, Mundy J, Ohashi Y, Walker JC (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7:301–308
Ishizaki-Nishizawa O, Fujii T, Azuma M, Sekiguchi K, Murata N, Ohtani T, Toguri T (1996) Low-temperature resistance of higher plants is significantly enhanced by a nonspecific cyanobacterial desaturase. Nat Biotechnol 14:1003–1006
Jin UH, Lee JW, Chung YS, Lee JH, Yi YB, Kim YK, Hyung NI, Pyee JH, Chung CH (2001) Characterization and temporal expression of a ω-6 fatty acid desaturase cDNA from sesame (Sesamum indicum L.) seeds. Plant Sci 161:935–941
Jonak C, Kiegerl S, Ligterink W, Barker PJ, Huskisson NS, Hirt H (1996) Stress signalling in plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proc Natl Acad Sci USA 93:11274–11279
Kaplan F, Guy CL (2004) β-Amylase induction and the protective role of maltose during temperature shock. Plant Physiol 135:1674–1684
Kim MC, Chung WS, Yun DJ, Cho MJ (2009) Calcium and calmodulin-mediated regulation of gene expression in plants. Mol Plant 2:13–21. doi:10.1093/mp/ssn091
Klimecka M, Muszyńska G (2007) Structure and functions of plant calcium-dependent protein kinases. Acta Biochim Pol 54:219–233
Knight H, Knight MR (2001) Abiotic stress signalling pathways: specificity and cross-talk. Trends Plant Sci 6:262–267
Knight H, Trewavas AJ, Knight MR (1996) Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after cold acclimation. Plant Cell 8:489–503
Knight H, Zarka DG, Okamoto H, Thomashow MF, Knight MR (2004) Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the CRT promoter element. Plant Physiol 135:1710–1717
Koo BC, Bushman BS, Mott IW (2008) Transcripts associated with non-acclimated freezing response in two barley cultivars. Plant Genome 1:21–32. doi:10.3835/plantgenome2008.06.0344
Kosova K, Vitamvas P, Prasil IT (2007) The role of dehydrins in plant response to cold. Biologia Plantarum 51:601–617. doi:10.1007/s10535-007-0133-6
Kosova K, Holkova L, Prasil IT, Prasilova P, Bradacova M, Vitamvas P, Capkova V (2008) Expression of dehydrin 5 during the development of frost tolerance in barley (Hordeum vulgare). J Plant Physiol 165:1142–1151
Kovacs D, Kalmar E, Torok Z, Tompa P (2008) Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol 147:381–390
Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, Harper JF (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol 130:2129–2141
Last RL, Jones AD, Shachar-Hill Y (2007) Towards the plant metabolome and beyond. Nat Rev Mol Cell Biol 8:167–174
Lee BH, Lee H, Xiong L, Zhu JK (2002) A mitochondrial complex I defect impairs cold-regulated nuclear gene expression. Plant Cell 14:1235–1251
Li W, Li M, Zhang W, Welti R, Wang X (2004) The plasma membrane-bound phospholipase Dδ enhances freezing tolerance in Arabidopsis thaliana. Nat Biotechnol 22:427–433
Liu HT, Li GL, Chang H, Sun DY, Zhou RG, Li B (2007) Calmodulin-binding protein phosphatase PP7 is involved in thermotolerance in Arabidopsis. Plant Cell Environ 30:156–164
Liu HT, Gao F, Li GL, Han JL, Liu DL, Sun DY, Zhou RG (2008) The calmodulin-binding protein kinase 3 is part of heat shock signal transduction in Arabidopsis thaliana. Plant J 55:760–773
Maali R, Schimschilaschvili HR, Pchelkin VP, Tsydendambaev VD, Nosov AM, Los DA, Goldenkova-Pavlova IV (2007) Comparative expression in Escherichia coli of the native and hybrid genes for acyl-lipid ∆9-desaturase. Russian J Genet 43:121–126. doi:10.1134/S1022795407020044
Maali-Amiri R, Goldenkova-Pavlova IV, Yur’eva NO, Pchelkin VP, Tsydendambaev VD, Vereshchagin AG, Deryabin AN, Trunova TI, Los DA, Nosov AM (2007) Lipid fatty acid composition of potato plants transformed with the ∆12-desaturase gene from cyanobacterium. Russian J Plant Physiol 54:600–606. doi:10.1134/S1021443707050056
Maeda H, Song W, Sage TL, DellaPenna D (2006) Tocopherols play a crucial role in low-temperature adaptation and phloem loading in Arabidopsis. Plant Cell 18:2710–2732
Matos AR, Hourton-Cabassa C, Cicek D, Reze N, Arrabaca JD, Zachowski A, Moreau F (2007) Alternative oxidase involvement in cold stress response of Arabidopsis thaliana fad2 and FAD3+ cell suspensions altered in membrane lipid composition. Plant Cell Physiol 48:856–865
McAinsh MR, Pittman JK (2009) Shaping the calcium signature. New Phytol 181:275–294
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Monroy AF, Dhindsa RS (1995) Low temperature signal transduction: induction of cold acclimation-specific genes of alfalfa by calcium at 25°C. Plant Cell 7:321–331
Monroy AF, Sangwan V, Dhindsa RS (1998) Low temperature signal transduction during cold acclimation: protein phosphatase 2A as an early target for cold-inactivation. Plant J 13:653–660
Murata N, Los DA (1997) Membrane fluidity and temperature perception. Plant Physiol 115:875–879
Murata N, Wada H (1995) Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of Cyanobacteria. Biochem J 308:1–8
Nakaminami K, Hill K, Perry SE, Sentoku N, Long JA, Karlson DT (2009) Arabidopsis cold shock domain proteins: relationships to floral and silique development. J Exp Bot 60:1047–1062
Nakashima K, Yamaguchi-Shinozaki K (2006) Regulons involved in osmotic stress-responsive and cold stress-responsive gene expression in plants. Plant Physiol 126:62–71. doi:10.1111/j.1399-3054.2005.00592.x
Nakashima K, Ito Y, Yamaguchi-Shinozaki K (2009) Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol 149:88–95
Nguema-Ona E, Bannigan A, Chevalier L, Baskin TI, Driouich A (2007) Disruption of arabinogalactan proteins disorganizes cortical microtubules in the root of Arabidopsis thaliana. Plant J 52:240–251
Nover L, Scharf KD (1997) Heat stress proteins and transcription factors. Cell Mol Life Sci 53:80–103
Novillo F, Alonso JM, Ecker JR, Salinas J (2004) CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. Proc Natl Acad Sci USA 101:3985–3990. doi:10.1073/pnas.0303029101
Orlova IV, Serebriiskaya TS, Popov V, Merkulova N, Nosov AM, Trunova TI, Tsydendambaev VD, Los DA (2003) Transformation of tobacco with a gene for the thermophilic acyl-lipid desaturase enhances the chilling tolerance of plants. Plant Cell Physiol 44:447–450
Örvar BL, Sangwan V, Omann F, Dhindsa RS (2000) Early steps in cold sensing by plant cells: the role of actin cytoskeleton and membrane fluidity. Plant J 23:785–794
Ouellet F (2007) Cold acclimation and freezing tolerance in plants. Encycl Life Sci. doi:10.1002/9780470015902.a0020093
Park SJ, Kwak KJ, Oh TR, Kim YO, Kang H (2009) Cold shock domain proteins affect seed germination and growth of Arabidopsis thaliana under abiotic stress conditions. Plant Cell Physiol 50:869–878
Plieth C, Hansen UP, Knight H, Knight MR (1999) Temperature sensing by plants: the primary characteristics of signal perception and calcium response. Plant J 18:491–497
Pourtau N, Jennings R, Pelzer E, Pallas J, Wingler A (2006) Effect of sugar-induced senescence on gene expression and implications for the regulation of senescence in Arabidopsis. Planta 224:556–568
Rapacz M, Wolanin B, Hura K, Tyrka M (2008) The effects of cold acclimation on photosynthetic apparatus and the expression of COR14b in four genotypes of barley (Hordeum vulgare) contrasting in their tolerance to freezing and high-light treatment in cold conditions. Ann Bot 101:689–699
Rekarte-Cowie I, Ebshish OS, Mohamed KS, Pearce RS (2008) Sucrose helps regulate cold acclimation of Arabidopsis thaliana. J Exp Bot 59:4205–4217
Renaut J, Hausman JF, Bassett C, Artlip T, Cauchie HM, Witters E, Wisniewski M (2008) Quantitative proteomic analysis of short photoperiod and low-temperature responses in bark tissues of peach (Prunus persica L. Batsch). Tree Genet Genomes 4:589–600. doi:10.1007/s11295-008-0134-4
Rorat T (2006) Plant dehydrins—tissue location, structure and function. Cell Mol Biol Lett 11:536–556
Russel NJ (1984) Mechanism of thermal adaptation in bacteria: blueprints for survival. Trends Biochem Sci 9:108–112
Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell 14:S401–S417
Sangwan V, Foulds I, Singh J, Dhindsa RS (2001) Cold-activation of Brassica napus BN115 promoter is mediated by structural changes in membranes and cytoskeleton, and requires Ca2+ influx. Plant J 27:1–12
Sangwan V, Örvar BL, Beyerly J, Hirt H, Dhindsa RS (2002) Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J 31:629–638
Seo PJ, Lee AK, Xiang F, Park CM (2008) Molecular and functional profiling of Arabidopsis pathogenesis-related genes: insights into their roles in salt response of seed germination. Plant Cell Physiol 49:334–344
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Somerville J (1999) Activities of cold-shock domain proteins in translation control. BioEssays 21:319–325
Somerville C, Browse J (1991) Plant lipids: metabolism, mutants, and membranes. Science 252:80–87
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94:1035–1040
Sugie A, Naydenov N, Mizuno N, Nakamura C, Takumi S (2006) Overexpression of wheat alternative oxidase gene Waox1a alters respiration capacity and response to reactive oxygen species under low temperature in transgenic Arabidopsis. Genes Genet Syst 81:349–354
Svensson JT, Crosatti C, Campoli C, Bassi R, Stanca AM, Close TJ, Cattivelli L (2006) Transcriptome analysis of cold acclimation in barley albina and xantha mutants. Plant Physiol 141:257–270
Tähtiharju S, Palva T (2001) Antisense inhibition of protein phosphatase 2C accelerates cold acclimation in Arabidopsis thaliana. Plant J 26:461–470
Teige M, Scheikl E, Eulgem T, Doczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H (2004) The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell 15:141–152
Thion L, Mazars C, Thuleau P, Graziana A, Rossignol M, Moreau M, Ranjeva R (1996) Activation of plasma membrane voltage-dependent calcium-permeable channels by disruption of microtubules in carrot cells. FEBS Lett 393:13–18
Thomashow MF (2001) So what’s new in the field of plant cold acclimation? lots!. Plant Physiol 125:89–93
Thomson GAJR (1989) Membrane acclimation by unicellular organisms in response to temperature change. J Bioenerg Biomembr 21:43–60
Townley HE, Knight MR (2002) Calmodulin as a negative regulator of Arabidopsis COR gene expression. Plant Physiol 128:1169–1172
Uemura M, Steponkus PL (2003) Modification of the intracellular sugar content alters the incidence of freeze-induced membrane lesions of protoplasts isolated from Arabidopsis thaliana leaves. Plant Cell Environ 26:1083–1096. doi:10.1046/j.1365-3040.2003.01033.x
Uemura M, Joseph RA, Steponkus PL (1995) Cold acclimation of Arabidopsis thaliana (effect on plasma membrane lipid composition and freeze-lnduced lesions). Plant Physiol 109:15–30
Uemura M, Tominaga Y, Nakagawara C, Shigematsu S, Minami A, Kawamura Y (2006) Responses of the plasma membrane to low temperatures. Physiol Plant 126:81–89. doi:10.1111/j.1399-3054.2005.00594.x
Ukaji N, Kuwabara C, Takezawa D, Arakawa K, Yoshida S, Fujikawa S (1999) Accumulation of small heat-shock protein homologs in the endoplasmic reticulum of cortical parenchyma cells in mulberry in association with seasonal cold acclimation. Plant Physiol 120:481–489
Vaultier MN, Cantrel C, Vergnolle C, Justin AM, Demandre C, Benhassaine-Kesri G, Cicek D, Zachowski A, Ruelland E (2006) Desaturase mutants reveal that membrane rigidification acts as a cold perception mechanism upstream of the diacylglycerol kinase pathway in Arabidopsis cells. FEBS Lett 580:4218–4223
Vergnolle C, Vaultier MN, Taconnat L, Renou JP, Kader JC, Zachowski A, Ruelland E (2005) The cold-induced early activation of phospholipase C and D pathways determines the response of two distinct clusters of genes in Arabidopsis cell suspensions. Plant Physiol 139:1217–1233
Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF (2005) Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. Plant J 41:195–211
Wada H, Gombos Z, Murata N (1994) Contribution of membrane lipids to the ability of the photosynthetic machinery to tolerate temperature stress. Proc Natl Acad Sci USA 91:4273–4277
Webb MS, Steponkus PL (1993) Freeze-lnduced membrane ultrastructural alterations in rye (Sede cereale) leaves. Plant Physiol 101:955–963
Webb MS, Gilmour SJ, Thomashow MF, Steponkus PL (1996) Effects of COR6.6 and COR15am polypeptides encoded by COR (cold-regulated) genes of Arabidopsis thaliana on dehydration-lnduced phase transitions of phospholipid membranes. Plant Physiol 111:301–312
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14:S165–S183
Yan SP, Zhang QY, Tang ZC, Su WA, Sun WN (2006) Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol Cell Proteomics 5:484–496
Yano R, Nakamura M, Yoneyama T, Nishida I (2005) Starch-related α-glucan/water dikinase is involved in the cold-induced development of freezing tolerance in Arabidopsis. Plant Physiol 138:837–846
Yoshida K, Terashima I, Noguchi K (2007) Up-regulation of mitochondrial alternative oxidase concomitant with chloroplast over-reduction by excess light. Plant Cell Physiol 48:606–614
Zhu J, Dong CH, Zhu JK (2007) Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr Opin Plant Biol 10:290–295
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Kononowicz.
Rights and permissions
About this article
Cite this article
Heidarvand, L., Maali Amiri, R. What happens in plant molecular responses to cold stress?. Acta Physiol Plant 32, 419–431 (2010). https://doi.org/10.1007/s11738-009-0451-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-009-0451-8