Abstract
Barley (Hordeum vulgare) seedlings were treated with spermidine prior to water deficit to determine whether this polyamine is able to affect the activity of superoxide dismutase -SOD (EC 1.15.1.1) responsible for hydrogen peroxide and superoxide radical level.
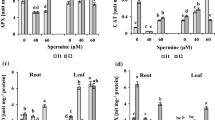
Short-term dehydration (24h) resulted in decrease of the SOD specific activity and a distinct increase in the superoxide anion and hydrogen peroxide contents. Polyamine treatment caused a substantial reduction in the contents of these two stress-raised reactive oxygen species and thereby lowered the oxidative stress in plant cells. Antioxidant system as an important component of the water-stress-protective mechanism can be changed by polyamines, able to moderate the radical scavenging system and to lessen in this way the oxidative stress.
Similar content being viewed by others
References
Acar O., Türkan I., Özdemir F. 2001. Superoxide dismutase and peroxidase activities in drought sensitive and resistant barley (Hordeum vulgare L.) varieties. Acta. Physiol. Plant. 23: 351–356.
de Agazio M. Zacchini M., Federico R., Grego S. 1995. Putrescine accumulation in maize roots treated with spermidine: evidence for spermidine to putrescine conversion. Plant Sci. 111: 181–185.
Ahuja B.S., Kaur K. 1985. Alteration in superoxide dismutase, peroxidase, lipid peroxidation and non-protein SH content in mung bean (Vigna radiata) seedlings subjected to water stress. Indian J. Exp. Biol. 23: 57–59.
Allen R.D. 1995. Dissection of oxidative stress toleramce using transgenic plants. Plant Physiol. 107: 1049–1054.
Alscher R.G., Erturk N., Heath L.S. 2002. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 53: 1331–1341.
Alscher R.G., Hess J.L. 1993. Antioxidants in higher plants. Boca Raton: CRC Press.
Allen R.D. 1995. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 107: 1049–1054.
Badiani M., De Biasi M.G., Artemi F. 1990. Catalase, peroxidase and superoxide dismutase activities in seedlings submitted to increasing water deficit. Agrochimica 34: 90–102.
Beauchamp C., Fridovich I. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 44: 276–278.
Besford R.T., Richardson J.L., Campos A.F., Tiburcio A.F. 1993. Effect of polyamines in stabilization of molecular complexes of thylakoid membranes of osmotically stressed oat leaves. Planta 189: 201–206.
Bolkhina O., Virolainen E., Fagerstedt K.V. 2003. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: a Review. Ann. Bot. 91: 179–194.
Bors N., Langebartels C., Michel C., Sanderman H. Jr 1989. Polyamines as radical scavengers and protectants against ozone damage. Phytochemistry 28: 1589–1595.
Borrell A., Carbonell R., Farras R., Puig-Parellada P., Tiburcio A.F. 1987. Polyamines inhibit lipid peroxidation in senescing oat leaves. Physiol. Plant. 99: 385–390.
Bowler Ch., Van Montagu M., Inzé D. 1992. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43: 83–116.
Bradford M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Bratton D.L. 1994. Polyamine inhibition of transbilayer movements of plasma membrane phospholipids in the erythrocyte ghost. 1994. J. Biol. Chem. 269: 22517–22523.
Bouchereau A., Aziz A., Larher F., Martin-Tanguy J. 1999. Polyamines and environmental challenges: recent development. Plant Sci. 140: 103–125.
Chandru H.K., Kim E., Kuk Y., Cho K., Han O. 2003. Kinetics of wound-induced activation of antioxidative enzymes in Oriza sativa: differential activation at different growth stages. Plant Sci. 164: 935–941.
del Río L.A., Corpas F.J., Sandalio L.M., Palma J.M., Gomez M. Barroso J.B. 2002. reactive oxygen species, antioxidant system and nitric oxide in peroxysomes. J. Exp. Bot. 53: 1255–1272.
Doke N. 1983. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophtora infestans and the hyphal wall components. Physiol. Plant Pathol. 23: 345–357.
Droillard M.J., Paulin A. 1990. Isozymes of superoxide dismutase in mitochondria and peroxisomes isolated from carnation (Dianthus caryophyllus) during senescence. Plant Physiol. 94: 1187–1192.
Elstner E.F. 1991. Mechanisms in oxygen activation in different compartments of plant cells. In: Pell E.J., Steffen K.L., eds. Active oxygen/oxidative stress and plant metabolism. Rockville, MD: American Society of Plant Physiologists, 13–25.
Filek M., B czek R., Niewiadomska E., Pilipowicz M., Ko cielniak J. 1997. Effect of high temperature treatment of Vicia faba roots on the oxidative stress enzymes in leaves. Acta Bioch. Pol. 44: 315–322.
Fridovich I. 1986a. Superoxide dismutases. Advances in Enzymology and Related Areas of Molecular Biology 58: 61–97.
Fridovich I. 1986b. Biological effects of superoxide radical. Arch. Biochem. Biophys. 247: 1–11.
Flores H.E., Glaston A.W. 1984a. Osmotic stress-induced polyamine accumulation in cereal leaves. I. Physiological parameters of the response. Plant Physiol. 75: 102–109.
Flores H.E., Galston A.W. 1984b. Osmotic stress-induced polyamine accumulation in cereal leaves. II. Relation to amino acid pools. Plant Physiol. 75: 110–113.
Halliwell B. 1987. Oxidative damage, lipid peroxidation and antioxidant protection in chloroplasts. Chem. Phys. Lipids 44: 327–340.
Jagtap V., Bhargava S. 1995. Variation in the antioxidant metabolism of drought-tolerant and drought susceptible varieties of Sorghum bicolor (L.) Moench. exposed to high light, low water and high temperature stress. J. Plant Physiol. 145: 195–197.
Kakkar R.K., Sawhney V.K. 2002. Polyamine research in plants — a changing perspective. Physiol. Plant. 116: 281–292.
Kubi J. 2001. Polyamines and “scavenging system”: influence of exogenous spermidine on Halliwell-Asada pathway enzyme activity in barley leaves under water deficit. Acta Physiol. Plant. 23: 335–341.
Kubi J. 2003. Polyamines and “scavenging system”: influence of exogenous spermidine on catalase and guaiacol peroxidase activities, and free polyamines level in barley leaves under water deficit. Acta Physiol. Plant. 25: 337–343.
León J., Rojo E., Sánchez-Serrano J.J. 2001. Wound signaling in plants. J. Exp. Bot. 52: 1–9.
Lester G.E. 2000. Polyamines and their cellular anti-senescence properties in honey dew muskmelon fruit. Plant Sci. 160: 105–112.
Levine A., Tenhaken R., Dixon R., Lamb C. 1994. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593.
McCord J.M., Fridovich I. 1969. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244: 6049–55.
Messner B., Boll M. 1994. Cell suspension cultures of spruce (Picea abies): inactivation of extracellular enzymes by fungal elicitor-induced transient release of hydrogen peroxide. Plant Cell Tissue and Organ Culture 39: 69–78.
Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 9: 405–410.
Navari-Izzo F., Pinzino C., Quartacci M.F., Sgherri C.L.M. 1994. Intracellular membranes: kinetics of superoxide production and changes in thylakoids of resurrection plants upon dehydration and rehydration. Proc. Roy. Soc. Edin. 102: 187–191.
Neill S.J., Desikan R., Clarke A., Hurst R.D., Hancock J. 2002. Hydrogen peroxide and nitric oxide as signaling molecules in plants. J. Exp. Bot. 53: 1237–1247.
Quartacci M.F., Navari-Izzo F. 1992. Water stress and free radical mediated changes in sunflower seedlings. J. Plant Physiol. 139: 621–625.
Robinson J.M. 1988. Does oxygen photoreduction occur in vivo? Physiol. Plant. 72: 666–680.
Sairam R.K., Srivastava G.C. 2001. Water stress tolerance of wheat (Triticum aestivum L.): Variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J. Agron. Crop Sci. 186: 63–70
Shen W., Nada K., Tachibana S. 2000. Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiol. 124: 431–439.
Smirnoff N. 1993. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 125: 27–58.
Tiburico A.F., Kaur-Sawhney R., Galston A. W. 1990. Polyamine metabolism of plants, in: B.J. Miflin P.J. Lea (eds.), The Biochemistry of Plants, vol. 16, Academic Press, New York, pp. 283–325.
Turner L.B., Stewart G.R. 1986. The effect or water stress upon polyamine levels in barley (Hordeum vulgare L.) leaves. J. Exp. Bot. 175: 170–177.
Turner L.B., Stewart G.R. 1988. Factors affecting polyamine accumulation in barley (Hordeum vulgare L.) leaf sections during osmotic stress. J. Exp. Bot. 200: 311–316.
Takahashi M.A., Asada K. 1983. Superoxide anion permeability of phospholipid membranes and chloroplast thylacoids. Arch. Biochem. Biophys. 226: 558–566.
Vranová E., Inzé D., Van Breusegem F. 2002. Signal transduction during oxidative stress. J. Exp. Bot. 372: 1227–1236.
Weatherley P.E. 1950. Studies in water relations of cotton plants. I. The field measurement of water deficits in leaves. New Phytol. 49: 81–97.
Zhang X., Zhang L., Dong F., Gao J., Galbraith D.W., Song C.P. 2001. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 126: 1438–1448.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kubi, J. The effect of exogenous spermidine on superoxide dismutase activity, H2O2 and superoxide radical level in barley leaves under water deficit conditions. Acta Physiol Plant 27, 289–295 (2005). https://doi.org/10.1007/s11738-005-0005-7
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-005-0005-7