Abstract
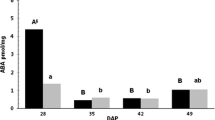
Changes in the abscisic acid (ABA) levels in embryo axes of seeds, belonging to the orthodox (Norway maple — Acer platanoides L.) and recalcitrant (sycamore — Acer pseudoplatanus L.) categories, were investigated throughout maturation using an ELISA (enzyme-linked immunosorbent assay) test. Concentration of ABA in embryo axes substantially differed depending on species and sampling date. ABA was always higher in Norway maple except at the end of seed maturation when ABA content was similar in both species. During maturation ABA decreased in both species but the decline was more marked in Norway maple than in sycamore (11 vs. 3 fold). These species also differed in the pattern of ABA changes, which in sycamore embryo axes was very regular, while in Norway maple a sharp decrease was recorded after acquisition by the seeds of tolerance to desiccation. Dehydration of embryo axes of Norway maple caused a further significant decrease of ABA level. In contrast, in dehydrated sycamore embryo axes ABA content did not decrease, but slightly increased. The role of ABA in desiccation tolerance and dormancy of Norway maple and sycamore seeds is discussed.
Similar content being viewed by others
References
Ackerson R. 1984. Regulation of soybean embryogenesis by abscisic acid. J. Exp. Bot. 34: 403–413.
Bewley J.D., Black S. 1982. Physiology and Biochemistry of Seeds in Relation to Germination. vol. 2, pp. 60–125, Springer-Verlag, Berlin.
Black M. 1991. Involvement of ABA in the physiology of developing and mature seeds. In: Abscisic Acid. Physiology and Biochmistry, ed. by W.J. Davies, H.G. Jones, Oxford: Bios Sci. Publ. Ltd: 99–124
Blackman S.A., Wettlaufer S.H., Obendorf R.L., Leopold A.C. 1991. Maturation proteins associated with desiccation tolerance in soybean. Plant Physiol. 96: 868–874.
Dickie J.B., May K., Morris S.V.A., Titley S.E. 1991. The effect of desiccation on seed survival in Acer platanoides L. and Acer pseudoplatanus L. Seed Sci. Res. 1: 149–162.
Finch-Savage W.E., Pramanik S.K., Bewley J.D. 1994. The expression of dehydrin proteins in desiccation-sensitive (recalcitrant) seeds of temperate trees. Planta 193: 478–485.
Harada J.J. 1997. Seed maturation and control of germination. In: Cellular and Molecular Biology of Plant Seed Development, ed. by B.A. Larkins, I.K. Vasil, Kluwer Acad. Publ.: 545–592.
Hong T.D., Ellis R.H. 1990. A comparison of maturation drying, germination and desiccation tolerance between developing seeds of Acer pseudoplatanus L. and Acer platanoides L. New Phytol. 116: 589–596.
Hsu F.C. 1979. Abscisic acid accumulation in developing seeds of Phaseolus vulgaris L. Plant Physiol. 63: 552–556.
Kermode A.R., Dumbroff E.B., Bewley J.D. 1989. The role of maturation drying in the transition from seed development to germination. VII. Effects of partial and complete desiccation on abscisic acid levels and sensitivity in Ricinus communis L. seeds. J. Exp.Bot. 40: 303–313.
Leon-Kloosterziel K.M., van de Bunt G.A., Zeevaart J.A.D., Koornneef M. 1996. Arabidopsis mutants with a reduced seed dormancy. Plant Physiol. 110: 233–240.
Mertens R., Deus-Neumann B., Weiler E.W. 1983. Monoclonal antibodies for the detection and quantification of the endogenous plant growth regulator, abscisic acid. FEBS Letters 160: 269–272.
Ooms J.J.J., Leon-Kloosterziel K.M., Bartels D., Koornneef M., Karssen C.M. 1993. Acquisition of desiccation tolerance and longevity in seeds of Arabidopsis thaliana. Plant Physiol. 102: 1185–1191.
Pinfield N.J., Dungey N.O. 1985. Seed dormancy in Acer: an assessment of the role of the structures covering the embryo. J. Plant Physiol. 120: 65–81.
Pinfield N.J., Stutchbury P.A., Bazaid S.A. 1987. Seed dormancy in Acer: is there a common mechanism for all Acer species and what part is played in it by abscisic acid. Physiol. Plant. 71: 365–371.
Pinfield N.J., Stutchbury P.A., Bazaid S.A., Gwarazimba V.E.E. 1989. Seed dormancy in Acer: the relationship between seed dormancy, embryo dormancy and abscisic acid in Acer plantanoides L. J. Plant Physiol. 135: 313–318.
Pinfield N.J., Stutchbury P.A., Bazaid S.A., Gwarazimba V.E.E. 1990. Abscisic acid and the regulation of embryo dormancy in the genus Acer. Tree Physiol. 6: 79–85.
Pukacka S. 1998. Carbohydrate content and sucrose metabolism in Acer platanoides and Acer pseudoplatanus seeds in relation to desiccation tolerance. Bulgar. Plant Physiol. Special issue, pp. 25.
Pukacka S., Czubak A. 1998. The effect of desiccation on viability and membrane lipid composition of Acer pseudoplatanus seeds. Acta Soc.Bot. Polon. 67: 249–252.
Pukacka S., Pukacki P.M. 1997. Changes in soluble sugars in relation to desiccation tolerance and effect of dehydration on freezing characteristics of Acer platanoides and Acer pseudoplatanus seeds. Acta Physiol. Plant. 19: 147–154.
Pukacka S., Wójkiewicz E. 2002. Carbohydrate metabolism in Norway maple and sycamore seeds in relation to desiccation tolerance. J Plant Physiol. 159: 273–279.
Rock C.D., Quatrano R.S. 1995. The role of hormones during seed development. In: Plant Hormones, ed. by P.J. Davies, Kluwer, Dordrecht.: 671–697.
Schussler J.R., Brenner M.L., Brun W.A. 1991. Relationship of endogenous abscisic acid to sucrose level and seed growth rate of soybeans. Plant Physiol. 96:1308–1313.
Singh Z., Browning G. 1991. The role of ABA in the control of apple seed dormancy re-appraised by combined gas chromatography-mass spectrometry. J. Exp. Bot. 42: 269–275.
Skriver K., Mundy J. 1990. Gene expression in response to abscisic acid and osmotic stress. Plant Cell 2: 503–512.
Walker-Simmons M. 1987. ABA levels and sensitivity in developing wheat embryos of sprouting resistant and susceptible cultivars. Plant Physiol. 84: 61–66.
Weiler E.W. 1982. An enzyme-immunoassay for cis-(+)-abscisic acid. Physiol. Plant. 54: 510–514.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pukacka, S., Gawrońska, H. Changes in abscisic acid levels in embryo axes of Norway maple and sycamore seeds during maturation and dehydration. Acta Physiol Plant 24, 149–155 (2002). https://doi.org/10.1007/s11738-002-0005-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-002-0005-9