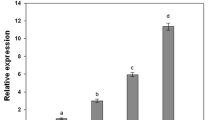
Abstract
Accumulation of some proteins isolated from the cell wall of roots of the Al-sensitive (Alfor) and the Al-resistant (Bavaria) barley cultivars were followed during treatment with different Al3+ concentrations, pH changes of the root medium, and several heavy metals (Cu2+, Cd2+, Co2+). SDS-PAGE analysis revealed an Al-induced accumulation of polypeptides with molecular mass of 14, and 16 kDa and a group of polypeptides around 27 kDa. The accumulation pattern of Al-induced polypeptides was very similar in both cultivars but in the Al-resistant Bavaria it was induced at lower Al concentration and earlier than it was in the Al-sensitive cultivar Alfor. Changes in pH values of root medium (pH 3.5–6.5) did not show any effect on the accumulation of Al-induced cell wall polypeptides either in Al-sensitive or in Al-tolerant barley cultivar. Heavy metals (Cu, Cd, and Co) at concentration of 10 µM resulted in similar accumulation of individual polypeptides as we found after Al treatment. In comparison to Al, quantitative differences in polypeptides accumulation induced by Cu, Cd and Co were less expressed that of Al treatment. More pronounced accumulation and earlier induction of individual cell wall polypeptides in roots of Al-resistant barley cultivar than in Al-sensitive, might indicate some possible role of these polypeptides in plant resistance to Al stress.
Similar content being viewed by others
Abbreviations
- BSA:
-
bovine serum albumine
- DTT:
-
dithiothreitol
- EGTA:
-
ethyleneglycolbis-(-amino-ethyl ether) N, N′-tetraacetic acid
- PVP:
-
polyvinylpyrrolidone
- SHAM:
-
salicylhydroxamic acid
References
Basu U, Basu A, Taylor GJ. 1994. Differential exudation of polypeptides by roots of aluminum-resistant and aluminum sensitive cultivars of Triticum aestivum L. in response to aluminum stress. Plant Physiol. 106: 151–158.
Basu U, Good AG, Aung T, Slaski JJ, Basu A, Briggs KG, Taylor GJ. 1999. A 23-kDa, root exudate polypeptide co-segregates with aluminum resistance in Triticum aestivum. Physiol. Plantarum 106: 53–61.
Basu U, Godbold D, Taylor GJ. 1994. Aluminum resistance in Triticum aestivum associated with enhanced exudation of malate. J. Plant Physiol. 144: 747–753.
Blamey FPC, Edmeades DC, Wheeler DM. 1990. Role of cation-exchange capacity in differential aluminum tolerance of Lotus species. J. Plant Nutr. 13: 729–744.
Cackmak I, Horst WJ. 1991. Effect of aluminium on net efflux of nitrate and potassium from root tips od soybean (Glycine max L.). J. Plant. Physiol. 138: 400–403.
Delhaize E, Ryan PR, Randall, PJ. 1993. Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol. 103: 695–702.
Delhaize E, Ryan PR. 1995. Aluminum toxicity and tolerance in plants. Plant Physiol. 107: 315–321.
Ezaki B, Sivaguru M, Ezaki, Y, Matsumoto H, Gardner RC. 1999. Acquisition of aluminum tolerance in Saccharomyces cerevisiae by expression of the BCB or NtGDI1 gene derived from plants. FEMS Microbiol. Lett. 171: 81–87.
Ezaki B, Gardner RC, Ezaki Y, Matsumoto H. 2000. Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol. 122: 657–665.
Hamel F, Breton C, Houde M. 1998. Isolation and characterization of wheat aluminum-regulated genes: possible involvement of aluminum as a pathogenesis response elicitor. Planta 205: 531–538.
Haug A. 1984. Molecular aspects of aluminum toxicity. CRC Crit. Rev. Plant Sci. 1: 345–373.
Heukesloven J, Dernick, R. 1985. Simplified method for silver staining of proteins in polyacrylamide gels and the mechanism of silver stain. Electrophoresis 6: 103–112.
Horst JW. 1995. The role of the apoplast in aluminium toxicity and resistance of higher plants: a review. Z. Pflanzenernähr. Bodenk. 158: 419–428.
Horst WJ, Pschel AK, Schmohl N. 1997. Induction of callose formation is a sensitive marker for genotypic aluminium sensitivity in maize. Plant and Soil 192: 23–30.
Horst WJ, Schmohl N, Kollmeier M, Baluška F, Sivaguru M. 1999. Does aluminium affect root growth of maize through interaction with the cell wall — plasma membrane — cytoskeleton continuum. Plant and Soil 215: 163–174.
Huttová J, Tamás L, Mistrík I. 1998. Quantitative changes in maize cytoplasmic proteins induced by aluminium. Biol. Plant. 41: 547–554.
Jones DL, Kochian LV. 1995. Aluminum inhibition of the inositol 1,4,5,-triphosphate signal transduction pathway in wheat roots: A role in aluminum toxicity? Plant Cell 7: 1913–1922.
Kinraide TB, Ryan PR, Kochian LV. 1992. Interaction effect of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiol. 99: 1461–1468.
Kochian LV. 1995. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46: 237–260.
Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 277: 680–685.
Larsen PB, Degenhardt J, Tai C-Y, Stenzler LM, Howell SH, Kochian LV. 1998. Aluminum-resistant Arabidopsis mutatnts that exhibit altered pattern of aluminum accumulation and organic acid release from roots. Plant Physiol. 117: 9–18.
Lazof DB, Goldsmith JG, Rufy TW, Linton RW. 1994. Rapid uptake of aluminum into cells of intact soybean root tips. Plant Physiol. 106: 1107–1114.
Le Van H, Kuraishi S, Sakurai N. 1994. Aluminum-induced rapid root inhibition and changes in cell-wall components of squash seedlings. Plant Physiol. 106: 971–976.
Levi R, Wolf T, Fleminger G, Solomon B. 1998. Immuno detection of aluminium and aluminium induced conformational changes in calmodulin implications in Alzheimer’s disease. Mol. Cell. Biochem. 189: 41–46.
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275.
Ma JF, Taketa S, Yang ZM. 2000. Aluminum tolerance genes on the short arm of chromosome 3R are linked to organic acid release in triticale. Plant Physiol. 122: 687–694.
Macdiarmid CW, Gardner RC. 1998. Overexpression of the Saccharomyces cerevisiase magnesium transport system confers resistance to aluminum ion. J. Biol. Chem. 273: 1727–1732.
Marschner H. 1995. Mineral nutrition of higher plants. Academic Press — Harcourt Brace & Company, London
Miyasaka SC, Bute JG, Howell RK, Foy CD. 1991. Mechanism of aluminum tolerance in snapbean, root exudation of citric acid. Plant Physiol. 96: 737–743.
Olivetti GP, Cumming JR, Etherton B. 1995. Membrane potential depolarization of root cap cells precedes aluminum tolerance in snapbean. Plant Physiol. 109: 123–129.
Pellet DM, Grunes DL, Kochian LV. 1995. Organic acid exudation as an aluminum tolerance mechanism in maize (Zea mays L.). Planta 196: 788–795.
Pellet DM, Papernik LA, Kochian LV. 1996. Multiple aluminum-resistance mechanism in wheat. Roles of root apical phosphate and malata exudation. Plant Physiol. 112: 591–597.
Putterill J, Gardner R. 1988. Proteins with the potential to protect plants from Al toxicity. Biochim Biophys Acta 964: 137–145.
Ryan PR, Delhaize E, Randall PJ. 1995. Characterization of Al-stimulated efflux of malate from the apices of Al-tolerant wheat roots. Planta 196: 103–110.
Rengel Z. 1996. Uptake of aluminium by plant cell. New Phytol. 134: 389–406.
Rengel Z, Pińeros M, Tester M. 1995. Transmembrane calcium fluxes during Al stress. Plant and Soil 171: 125–131.
Rincón M, Gonzales RA. 1992. Aluminum partitioning in intact roots of aluminum-tolerant and aluminumsensitive wheat (Triticum aestivum L.) cultivars. Plant Physiol. 99: 1021–1028.
Slaski JJ. 1990. Response of calmodulin-dependent and calmodulin-independent NAD kinase to aluminum in root tips from various cultivated plants. J. Plant Physiol. 136: 40–44.
Snowden KC, Richards KD, Gardner RC. 1995. Aluminium-induced genes. Induction by toxic metals, low calcium, and wounding and pattern of expression in root tips. Plant Physiol. 107: 341–348.
Somers DJ, Briggs KG, Gustafson JP. 1996. Aluminum stress and protein synthesis in near isogenic lines of Triticum aestivum differing in aluminum tolerance. Physiol. Plantarum 97: 694–700.
Stass A, Horst WJ. 1995. Effect of aluminium on membrane properties of soybean (Glycine max) cells in suspension culture. Plant and Soil. 171: 113–118.
Sugimoto M, Sakamoto W. 1997. Putative phospholipid hydroperoxide glutathion peroxidase gene from Arabidopsis thaliana induced by oxidative stress. Genes Genet. Syst. 72: 311–316.
Tamás L, Mistrík I, Huttová J. 1999. Protein profiles in roots of aluminium sensitive and resistant barley cultivars after aluminium treatment. Biologia, Bratislava, 54: 459–465.
Vázquez MD, Poschenrieder C, Corrales I, Barcel J. 1999. Changes in apoplastic aluminum during the initial growth response to aluminum by roots of a tolerant maize variety. Plant Physiol. 119: 435–444.
You G, Nelson DJ. 1991. Al3+ versus Ca2+ ion binding to metionine and tyrosine spin-labeled bovine brain calmodulin. J. Inorg. Biochem. 41: 283–291.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tamás, L., Huttová, J., Hajasová, L. et al. The effect of aluminium on polypeptide pattern of cell wall proteins isolated from the roots of Al-sensitive and Al-resistant barley cultivars. Acta Physiol Plant 23, 161–168 (2001). https://doi.org/10.1007/s11738-001-0004-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11738-001-0004-2