Abstract
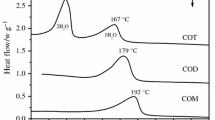
The dehydration processes of trisodium citrate (Na3C6H5O7) hydrates were investigated using thermogravimetry (TG), differential scanning calorimetry (DSC) and powder X-ray diffraction (PXRD). It was found that the temperature of dehydration of trisodium citrate dihydrate was at 430.99 K. For trisodium citrate pentahydrate, there is a two-step dehydration process and the endothermal peaks appear at 337.23 K and 433.83 K, respectively. During the first step of dehydration process, the structure of trisodium citrate pentahydrate changed into the structure of trisodium citrate dihydrate. In addition, the kinetics of dehydration for trisoidum citrate hydrates was also investigated using TG data. According to the activation energies of dehydration calculated by Ozawa equation, it was found that the dehydration mechanisms of the two hydrates were different.
Similar content being viewed by others
References
Bayes B, Bonal J, Romero R. Sodium citrate for filling haemodialysis catherters. Nephrology, Dialysis, Transplantation, 1999, 14(10): 2532–2533
Gao J Y, Xie C, Wang Y L, Xu Z, Hao H X. Solubility data of trisodium citrate hydrates in aqueous solution and crystal-solution interfacial energy of the pentahydrate. Crystal Research and Technology, 2012, 47(4): 397–403
Steiner T, Koellner G. Crystalline p-cyclodextrin hydrate at various humidities: fast, continuous, and reversible dehydration studied by X-ray diffraction. Journal of the American Chemical Society, 1994, 116(12): 5122–5128
Morris R E, Burton A, Bull L M, Zones S I. SSZ-51-A new aluminophosphate zeotype: synthesis, crystal structure, NMR, and dehydration properties. Chemistry of Materials, 2004, 16(15): 2844–2851
Boonchom B J, Vittayakorn N. Dehydration behavior of synthetic Al0.5Fe0.5PO4·2.5H2O. Journal of Chemical & Engineering Data, 2010, 55(9): 3307–3311
Frost R L, Kloprogge J T. Heating stage spectroscopy: infrared, Raman, energy dispersive X-ray and X-ray photoelectron spectroscopy. In: Brown M E, Gallagher P K, eds. Handbook of Thermal Analysis and Calorimetry. Oxford: Elsevier, 2008, 171–173
Sestak J. Thermodynamical Properties of Solids. Prague: Academia Publishers, 1984, 1–5
Young D. Decomposition of Solids. Oxford: Pergamon Press, 1966, 55–109
Arivanandhan M, Huang X M, Uda S, Bhagavannarayana G, Vijayan N, Sankaranarayanan K, Ramasamy P. Directional growth of organic NLO crystal by different growth methods: a comparative study by means of XRD, HRXRD and laser damage threshold. Journal of Crystal Growth, 2008, 310(21): 4587–4592
Duan Y, Li J, Yang X, Hu L, Wang Z, Liu Y, Wang C. Kinetic analysis on the non-isothermal dehydration by integral master-plots method and TG-FTIR study of zinc acetate dihydrate. Journal of Analytical and Applied Pyrolysis, 2008, 83(1): 1–6
Lee S B, Fasina O. Fasina O. TG-FTIR analysis of switchgrass pyrolysis. Journal of Analytical and Applied Pyrolysis, 2009, 86(1): 39–43
dos Santos A V, Matos J R. Dehydration studies of rare earth p-toluenesulfonate hydrates by TG/DTG and DSC. Journal of Alloys and Compounds, 2002, 344(1–2): 195–198
Yonemochi E, Hoshino T, Yoshihashi Y, Terada K. Evaluation of the physical stability and local crystallization of amorphous terfenadine using XRD-DSC and micro-TA. Thermochimica Acta, 2005, 432(1): 70–75
Fischer A, Palladino G. Trisodium citrate dihydrate. Acta Crystallographica. Section E, Structure Reports Online, 2003, 59(11): m1080–m1082
Voissat B, Rodier N. Crystal structure of sodium citrate hydrate. Bulletin de la Societe Chimique de France, 1986, 4: 522–525
Vyazovkin S. Isoconversional kinetics. In: Brown M E, Gallagher P K, eds. Handbook of Thermal Analysis and Calorimetry. Oxford: Elsevier, 2008, 504–506
Ozawa T. A new method of analyzing thermogravimetric data. Bulletin of the Chemical Society of Japan, 1965, 38(11): 1881–1886
Gamlin C D, Dutta N K, Choudhury N R. Evaluation of knietic parameters of thermal and oxidative decomposition of base oils by conventional, isothermal and modulated TGA, and pressure DSC. Thermochim Acta, 2002, 392–393: 357–369
Vyazovkin S. A unified approach to kinetic processing of nonisothermal data. International Journal of Chemical Kinetics, 1996, 28(2): 95–101
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, J., Wang, Y. & Hao, H. Investigations on dehydration processes of trisodium citrate hydrates. Front. Chem. Sci. Eng. 6, 276–281 (2012). https://doi.org/10.1007/s11705-012-1206-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-012-1206-4