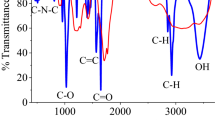
Abstract
The issue of residual heavy metals in environmental and water sources is a global challenge. Among heavy metals, chromium is a very dangerous metal that is very harmful to humans and all living things. Exposure may occur from natural or industrial sources of chromium. Therefore, designing an optical sensor to monitor Cr3+ ions in an aqueous solution with selectivity, simplicity, and high sensitivity is of great importance. In the present work, free radical polymerization is used to synthesize a magnetic Fe3O4@Pectin-polymethacrylamide@graphene quantum dot (Fe3O4@Pectin-MAM@GQD) nanocomposite with a core–shell structure. It is used as an optical nanosensor for the detection of Cr3+ ions in aqueous solutions. This method is based on increasing the UV–vis absorbance intensity of Fe3O4@Pectin-MAM@GQD in the presence of Cr3+ ions. At optimal conditions, pH = 6 and adsorbent dose of 0.3 mg/ml, the synthesized nanosensor showed a good linear correlation between the absorbance intensity of the nanosensor and the concentrations of Cr3+ in the range from 4 to 100 µM. Remarkably, the result showed that the LOD and LOQ of Fe3O4@Pectin-MAM@GQD nanocomposite are equal to 0.468 µM and 1.419 µM, receptively. Using this method can identify chromium in aqueous pollutants since a low limit of detection of chromium in aqueous solutions (MRL≈1 µM, WHO).







Similar content being viewed by others
References
Aladaghlo Z, Javanbakht S, Fakhari AR, Shaabani A (2021) Gelatin microsphere coated Fe3O4@ graphene quantum dots nanoparticles as a novel magnetic sorbent for ultrasound-assisted dispersive magnetic solid-phase extraction of tricyclic antidepressants in biological samples. Microchim Acta 188(3):1–9
Amini-Fazl MS, Barzegarzadeh M, Mohammadi R (2021) Surface modification of graphene oxide with crosslinked polymethacrylamide via RAFT polymerization strategy: effective removal of heavy metals from aqueous solutions. J Inorg Organomet Polym Mater 31:1–12
Anusuya T, Kumar V, Kumar V (2021) Hydrophilic graphene quantum dots as turn-off fluorescent nanoprobes for toxic heavy metal ions detection in aqueous media. Chemosphere 282:131019
Aroua MK, Leong S, Teo L, Yin CY, Daud WMAW (2008) Real-time determination of kinetics of adsorption of lead (II) onto palm shell-based activated carbon using ion selective electrode. Biores Technol 99(13):5786–5792
Bagbi Y, Pandey A, Solanki PR (2019) Electrospun nanofibrous filtration membranes for heavy metals and dye removal. Nanoscale materials in water purification. Elsevier, pp 275–288
Barzegarzadeh M, Amini-Fazl MS, Yazdi-Amirkhiz SY (2022) Polymethacrylamide-functionalized graphene oxide via the RAFT method: an efficient fluorescent nanosensor for heavy metals detection in aqueous media. Chem Pap 76:1–8
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J, Park J, Makino T, Kirkham MB, Scheckel K (2014) Remediation of heavy metal (loid) s contaminated soils–to mobilize or to immobilize? J Hazard Mater 266:141–166
Chahrour KM, Yam F, Eid A, Nazeer AA (2020) Enhanced photoelectrochemical properties of hierarchical black TiO2-x nanolaces for Cr (VI) photocatalytic reduction. Int J Hydrog Energy 45(43):22674–22690
Chen W, Li D, Tian L, Xiang W, Wang T, Hu W, Hu Y, Chen S, Chen J, Dai Z (2018) Synthesis of graphene quantum dots from natural polymer starch for cell imaging. Green Chem 20(19):4438–4442
Cheng H, Zhao Y, Fan Y, Xie X, Qu L, Shi G (2012) Graphene-quantum-dot assembled nanotubes: a new platform for efficient Raman enhancement. ACS Nano 6(3):2237–2244
Chu W, Zhang Y, Li D, Barrow CJ, Wang H, Yang W (2015) A biomimetic sensor for the detection of lead in water. Biosens Bioelectron 67:621–624
Dhar S, Majumder T, Mondal SP (2016) Graphene quantum dot-sensitized ZnO nanorod/polymer Schottky junction UV detector with superior external quantum efficiency, detectivity, and responsivity. ACS Appl Mater Interfaces 8(46):31822–31831
Du Y, Liu W, Qiang R, Wang Y, Han X, Ma J, Xu P (2014) Shell thickness-dependent microwave absorption of core–shell Fe3O4@ C composites. ACS Appl Mater Interfaces 6(15):12997–13006
Durgadas C, Sharma C, Sreenivasan K (2011) Fluorescent gold clusters as nanosensors for copper ions in live cells. Analyst 136(5):933–940
Ebrahim S, Shokry A, Khalil M, Ibrahim H, Soliman M (2020) Polyaniline/Ag nanoparticles/graphene oxide nanocomposite fluorescent sensor for recognition of chromium (VI) ions. Sci Rep 10(1):1–11
Faraz M, Abbasi A, Naqvi FK, Khare N, Prasad R, Barman I, Pandey R (2018) Polyindole/cadmium sulphide nanocomposite based turn-on, multi-ion fluorescence sensor for detection of Cr3+, Fe3+ and Sn2+ ions. Sens Actuators, B Chem 269:195–202
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92(3):407–418
Guo B, Liu Q, Su Q, Liu W, Ju P, Li G, Wu Q (2018) A triphenylamine-functionalized fluorescent organic polymer as a turn-on fluorescent sensor for Fe 3+ ion with high sensitivity and selectivity. J Mater Sci 53(22):15746–15756
Gao X, Du C, Zhuang Z, Chen W (2016) Carbon quantum dot-based nanoprobes for metal ion detection. J Mater Chem C 4(29):6927–6945
Guo Y, Zhang L, Zhang S, Yang Y, Chen X, Zhang M (2015) Fluorescent carbon nanoparticles for the fluorescent detection of metal ions. Biosens Bioelectron 63:61–71
Han MY, Özyilmaz B, Zhang Y, Kim P (2007) Energy band-gap engineering of graphene nanoribbons. Phys Rev Lett 98(20):206805
Hu C, Yu C, Li M, Wang X, Yang J, Zhao Z, Eychmüller A, Sun YP, Qiu J (2014) Chemically tailoring coal to fluorescent carbon dots with tuned size and their capacity for Cu (II) detection. Small 10(23):4926–4933
Jeong U, Shin HH, Kim Y (2015) Functionalized magnetic core–shell Fe@ SiO2 nanoparticles as recoverable colorimetric sensor for Co2+ ion. Chem Eng J 281:428–433
Jin W, Du H, Zheng S, Zhang Y (2016) Electrochemical processes for the environmental remediation of toxic Cr (VI): a review. Electrochim Acta 191:1044–1055
Ju P, Su Q, Liu Z, Li X, Guo B, Liu W, Li G, Wu Q (2019) A Salen-based covalent organic polymer as highly selective and sensitive fluorescent sensor for detection of Al 3+, Fe 3+ and Cu 2+ ions. J Mater Sci 54(1):851–861
Ju J, Zhang R, He S, Chen W (2014) Nitrogen-doped graphene quantum dots-based fluorescent probe for the sensitive turn-on detection of glutathione and its cellular imaging. RSC Adv 4(94):52583–52589
Kim H, Abdala AA, Macosko CW (2010) Graphene/polymer nanocomposites. Macromolecules 43(16):6515–6530
Kong L, Lu X, Bian X, Zhang W, Wang C (2011) Constructing carbon-coated Fe3O4 microspheres as antiacid and magnetic support for palladium nanoparticles for catalytic applications. ACS Appl Mater Interfaces 3(1):35–42
Kosynkin DV, Higginbotham AL, Sinitskii A, Lomeda JR, Dimiev A, Price BK, Tour JM (2009) Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458(7240):872–876
Kuilla T, Bhadra S, Yao D, Kim NH, Bose S, Lee JH (2010) Recent advances in graphene based polymer composites. Prog Polym Sci 35(11):1350–1375
Liu B, Zhang W, Yang F, Feng H, Yang X (2011) Facile method for synthesis of Fe3O4@ polymer microspheres and their application as magnetic support for loading metal nanoparticles. J Phys Chem C 115(32):15875–15884
Liu L, Cooke PH, Coffin DR, Fishman ML, Hicks KB (2004) Pectin and polyacrylamide composite hydrogels: effect of pectin on structural and dynamic mechanical properties. J Appl Polym Sci 92(3):1893–1901
Liu Y, Deng Y, Dong H, Liu K, He N (2017) Progress on sensors based on nanomaterials for rapid detection of heavy metal ions. Sci China Chem 60(3):329–337
Liu T, Li G, Zhang N, Chen Y (2012) An inorganic–organic hybrid optical sensor for heavy metal ion detection based on immobilizing 4-(2-pyridylazo)-resorcinol on functionalized HMS. J Hazard Mater 201:155–161
Lu J, Yeo PSE, Gan CK, Wu P, Loh KP (2011) Transforming C 60 molecules into graphene quantum dots. Nat Nanotechnol 6(4):247–252
Ma M, Zhang Y, Yu W, Shen H-y, Zhang H-q, Gu N (2003) Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids Surf A 212(2–3):219–226
Mackie ID, DiLabio GA (2008) Interactions in large, polyaromatic hydrocarbon dimers: application of density functional theory with dispersion corrections. J Phys Chem A 112(43):10968–10976
Mao Y, Bao Y, Han D, Li F, Niu L (2012) Efficient one-pot synthesis of molecularly imprinted silica nanospheres embedded carbon dots for fluorescent dopamine optosensing. Biosens Bioelectron 38(1):55–60
Mei Q, Zhang Z (2012) Photoluminescent graphene oxide ink to print sensors onto microporous membranes for versatile visualization bioassays. Angew Chem 124(23):5700–5704
Mohammadi R, Saboury A, Javanbakht S, Foroutan R, Shaabani A (2021) Carboxymethylcellulose/polyacrylic acid/starch-modified Fe3O4 interpenetrating magnetic nanocomposite hydrogel beads as pH-sensitive carrier for oral anticancer drug delivery system. Eur Polym J 153:110500
Mueller ML, Yan X, McGuire JA, Li L-s (2010) Triplet states and electronic relaxation in photoexcited graphene quantum dots. Nano Lett 10(7):2679–2682
Pan D, Zhang J, Li Z, Wu M (2010) Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv Mater 22(6):734–738
Parvin F, Rikta SY, Tareq SM (2019) Application of nanomaterials for the removal of heavy metal from wastewater. Nanotechnology in water and wastewater treatment. Elsevier, pp 137–157
Ponomarenko LA, Schedin F, Katsnelson MI, Yang R, Hill EW, Novoselov KS, Geim AK (2008) Chaotic dirac billiard in graphene quantum dots. Science 320(5874):356–358
Salimi F, Kiani M, Karami C, Taher MA (2018) Colorimetric sensor of detection of Cr (III) and Fe (II) ions in aqueous solutions using gold nanoparticles modified with methylene blue. Optik 158:813–825
Sang S, Zhang H, Sun Y, Jian A, Zhang W (2017) Facile synthesis of carbon-encapsulated Fe3O4 core/shell nanospheres for application in Pb (II) electrochemical determination. Int J Electrochem Sci 12(2):1306–1317
Shen J, Zhu Y, Yang X, Li C (2012) Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem Commun 48(31):3686–3699
Shrivastava A, Gupta VB (2011) Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron Young Sci 2(1):21–25
Traboulsi H, Awada C (2020) Toward the development of ultrasensitive detectors for environmental applications: a kinetic study of Cr (III) monitoring in water using EDTA and SERS techniques. ACS Omega 5(48):31352–31361
Waldron R (1955) Infrared spectra of ferrites. Phys Rev 99(6):1727
Wang W, Liu T, Yi D (2019) (2019) Detection of mercury ion based on quantum dots using miniaturised optical fibre sensor. J E 2019:8595–8598
Wang X, Boschetti C, Ruedas-Rama MJ, Tunnacliffe A, Hall EA (2010) Ratiometric pH-dot ANSors. Analyst 135(7):1585–1591
Werner S, Glaser C, Kasper T, Lê TNN, Gross S, Smarsly BM (2022) H2S Dosimetry by CuO: Towards stable sensors by unravelling the underlying solid‐state chemistry. Chem Eur J 28(3):202103437
Wu B-c, Degner B, McClements DJ (2014) Soft matter strategies for controlling food texture: formation of hydrogel particles by biopolymer complex coacervation. J Phys: Condens Matter 26(46):464104
Yan X, Cui X, Li L-s (2010) Synthesis of large, stable colloidal graphene quantum dots with tunable size. J Am Chem Soc 132(17):5944–5945
Yang W, Yang Y, Li H, Lin D, Yang W, Guo D, Pan Q (2020) Integration of Cd: ZnS QDs into ZIF-8 for enhanced selectivity toward Cu 2+ detection. Inorg Chem Front 7(19):3718–3726
Yeh Y-C, Creran B, Rotello VM (2012) Gold nanoparticles: preparation, properties, and applications in bionanotechnology. Nanoscale 4(6):1871–1880
Yu W, Sisi L, Haiyan Y, Jie L (2020) Progress in the functional modification of graphene/graphene oxide: a review. RSC Adv 10(26):15328–15345
Zhang X, Ren G, He Z, Yang W, Li H, Wang Y, Pan Q, Shi X (2020) Luminescent detection of Cr (VI) and Mn (VII) based on a stable supramolecular organic framework. Cryst Growth Des 20(10):6888–6895
Zhang Z, Zhang J, Chen N, Qu L (2012) Graphene quantum dots: an emerging material for energy-related applications and beyond. Energy Environ Sci 5(10):8869–8890
Zhao L, Wang J, Zhang P, Gu Q, Gao C (2018) Absorption of heavy metal ions by alginate. In: Bioactive seaweeds for food applications 255–268
Zhao D-L, Zhang H-L, Zeng X-W, Xia Q-S, Tang J-T (2006) Inductive heat property of Fe3O4/polymer composite nanoparticles in an ac magnetic field for localized hyperthermia. Biomed Mater 1(4):198
Acknowledgements
The authors express their sincere gratitude to the University of Tabriz for providing financial support to this research.
Funding
No funding resources.
Author information
Authors and Affiliations
Contributions
MB: Investigation, Software, Visualization, Methodology, Writing—Original Draft. MSAF: Conceptualization, Validation, Supervision, Resources, Writing—Review & editing. HN: Software, Visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barzegarzadeh, M., Amini-Fazl, M.S. & Nasrizadeh, H. A rapid and sensitive method to detection of Cr3+by using the Fe3O4@Pectin-polymethacrylimide@graphene quantum dot as a sensitive material. Chem. Pap. 77, 351–360 (2023). https://doi.org/10.1007/s11696-022-02484-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02484-7